Strategic Plan for Herpes Research Includes Vaccines
To advance the clinical research into herpes simplex virus (HSV) infections, the U.S. National Institutes of Health (NIH) today released the Strategic Plan for HSV.
An NIH-wide HSV Working Group was formed in October 2022 to develop this plan, informed by feedback from more than 100 representatives of the research and advocacy communities and stakeholders.
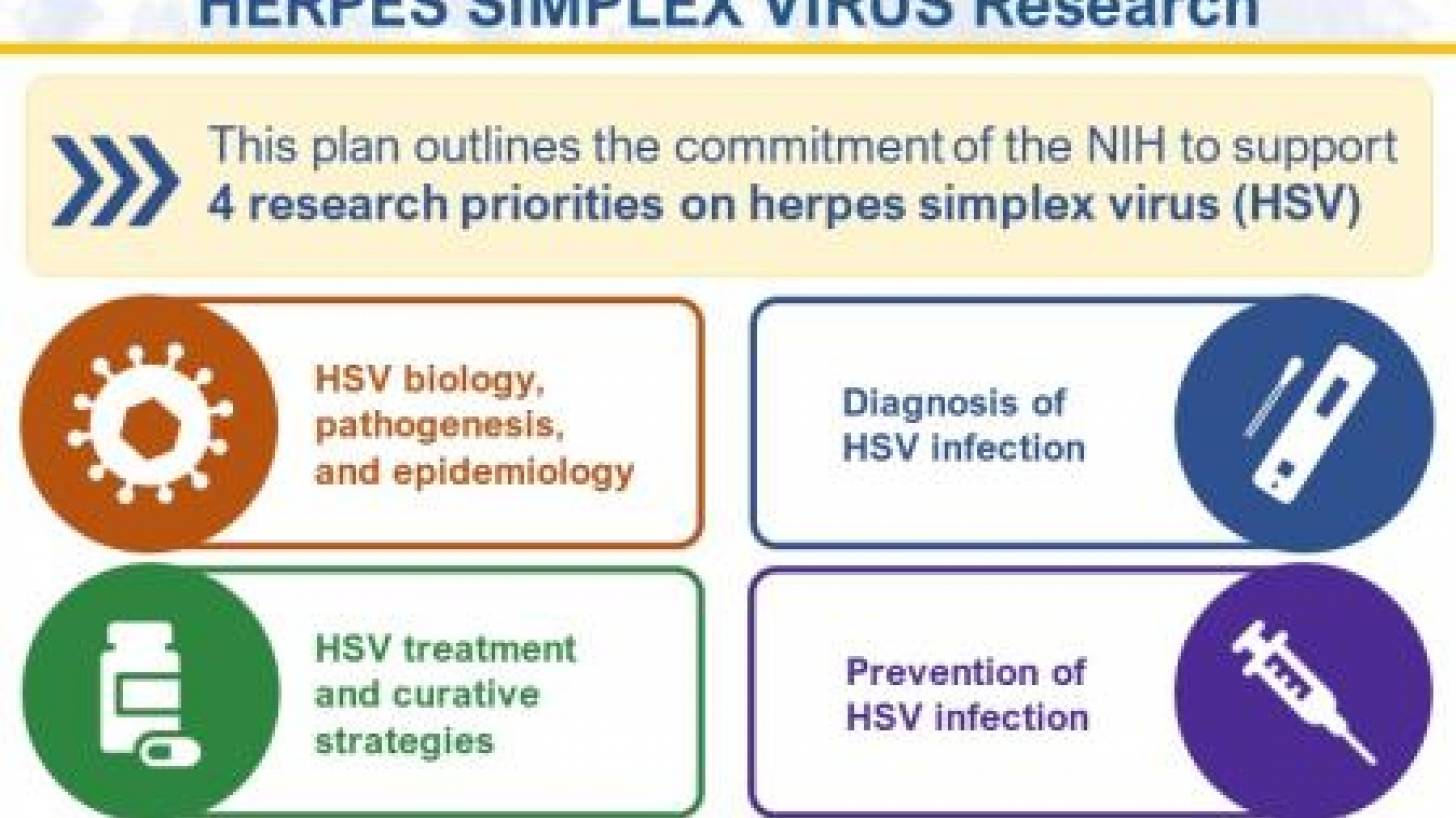
The innovative plan published on September 19, 2023, outlines an HSV research framework with four strategic priorities: improving fundamental knowledge of HSV biology, pathogenesis, and epidemiology; accelerating research to improve HSV diagnosis; improving strategies to treat HSV while seeking a curative therapeutic, and, advancing research to prevent HSV infection.
Additionally, this plan aligns with ongoing national efforts, including the Sexually Transmitted Infections National Strategic Plan.
To date, there are no HSV vaccines approved by the U.S. Food and Drug Administration (FDA).
While research advances have resulted in several therapeutics, the effectiveness of these treatments in reducing symptoms and viral transmission varies widely.
In addition, current treatment strategies require active virus replication, making them ineffective against latent HSV infection.
However, Moderna recently reaffirmed it is developing the mRNA-1608 vaccine candidate to reduce the burden of HSV lesions based on its world-class mRNA technology.
Moderna's mRNA platform offers potential advantages in efficacy, development speed, production scalability, and reliability.
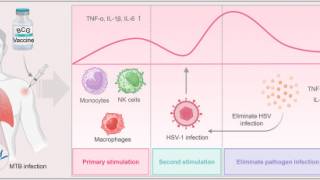
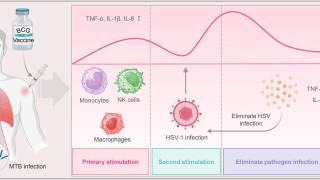
HSV can cause recurring blisters or sores, and in severe cases, HSV may lead to life-threatening or long-term complications, typically in the central nervous system. Neonatal herpes, if left untreated, is fatal in 60% of cases.
HSV-1 and HSV-2 are among the most common viral infections in the U.S.
Up to 80% of people between the ages of 14 and 49 years live with HSV-1, and more than 10% live with HSV-2, says the NIH.
Furthermore, HSV is a leading cause of viral encephalitis—brain inflammation from a viral infection—and infectious blindness worldwide.
The NIH anticipates that this plan will serve as a foundation for research, public health, and medical communities to work collaboratively to reduce the burden of HSV-1 and HSV-2.
Carolyn Deal, Ph.D., chief of the Enteric and Sexually Transmitted Infections Branch in NIAID's Division of Microbiology and Infectious Diseases, is available at [email protected] to comment on the significance and implementation of the plan.
Our Trust Standards: Medical Advisory Committee