U.S. NIH Herpes Strategic Plan Seeks Proposals

Over the past decade, the United States has witnessed alarming increases in rates of sexually transmitted infections (STIs), amounting to a public health crisis.
The consequences of the STI epidemic are enormous, wrote the U.S. HHS.
When left untreated, STIs can lead to long-term, irreversible health outcomes such as chronic pelvic pain, infertility, adverse pregnancy outcomes, neonatal death, and congenital abnormalities.
While there are vaccines for Hepatitis, HPV, Mpox, and many STIs lack preventive and/or therapeutic vaccines.
To address one STI, the National Institute of Allergy and Infectious Diseases (NIAID) recently issued a Notice announcing a Request for Information (RFI) inviting comments and suggestions on the U.S. National Institutes of Health's (NIH) key strategic approaches to develop a Herpes Simplex Virus (HSV) Strategic Plan.
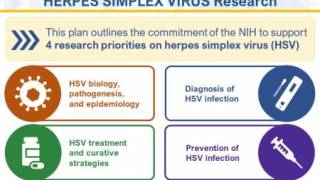
Announced on April 21, 2023, this RFI is an effort by multiple Institutes and Centers to enable the NIH's strategic plan to be structured around four research areas, as noted below.
Priority 1: Improve fundamental knowledge of HSV biology, pathogenesis, and epidemiology
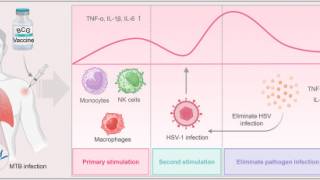
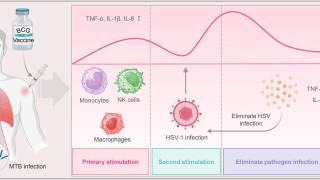
Enhance fundamental knowledge of HSV biology, including but not limited to viral interactions with host cells and mechanisms of replication and transport; fundamental aspects of innate and adaptive immune response to HSV; HSV disease pathogenesis in multiple organ systems, including the skin, reproductive tract, eye, and the peripheral and central nervous systems; and the critical drivers of disease transmission.
Characterize host and pathogen drivers that underlie dynamics of HSV latency and reactivation.
Improve understanding of diverse pathophysiology of HSV infection, including neonatal infection and the role of mucosal immunity to reduce genital and orolabial infection and disease; and a deeper understanding of the neurologic impact of HSV infection, including herpes simplex encephalitis, post-herpetic neuropathic pain, post-herpetic autoimmune encephalitis (i.e., post-HSV NMDARE), and potential associations with neurodegenerative disorders such as Alzheimer's disease.
Explore epidemiology of and co-morbidities associated with HSV infection
Improve and develop new in vitro and in vivo models that reflect human disease
Priority 2: Accelerate research to improve diagnosis by developing improved biomarkers and technologies for herpes diagnosis; Improve sensitivity and specificity of serologic tests that can be made commercially; Support research to improve point-of-care diagnostics.
Priority 3: Improve strategies to treat and cure HSV by identifying candidates for the elimination of virus or functional cure; Advance the development of novel treatment strategies, including strategies for preventing HSV entry into the central nervous system and for reducing sequelae of HSV encephalitis; Evaluate the safety and efficacy of treatment strategies in diverse populations and age groups; and Optimize therapy to minimize shedding and transmission.
Priority 4: Advance research to prevent HSV infection by supporting research to identify immune correlates of protection for HSV1 and HSV2; Advance the development of promising prophylactic vaccines; Support clinical trials to test new evaluation of therapies, diagnostics, and vaccines (therapeutic and prophylactic).
This NIAID RFI seeks input from stakeholders throughout the scientific research community and the general public regarding the proposed framework.
As of April 24, 2023, the U.S. FDA has not approved any herpes vaccine candidate.
Other STI vaccine news is posted by Precision Vaccinations.
Our Trust Standards: Medical Advisory Committee