$537,310 Funds HIV-Herpes Syndemic Study

While there are no HIV or Herpes vaccines approved for use in 2024, the U.S. National Institute of Allergy and Infectious Diseases recently awarded $537,310 to fund a Mechanisms Underlying the HIV-HSV-2 Syndemic study.
These sexually transmitted diseases are co-present in millions of individuals within the United States.
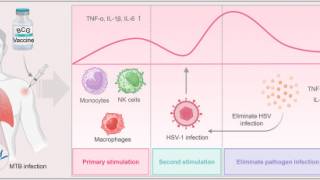
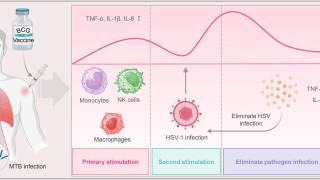
Award to Project Leader Betsy C. Herold and the Albert Einstein College of Medicine on February 20, 2024; this new study focuses on Herpes simplex virus type 2 (HSV-2) is a significant driver of the HIV epidemic, but the underlying biological mechanisms have not been delineated.
These researchers wrote, 'recurrent HSV-2 shedding, which occurs more often in HIV-infected persons, is characterized by the recruitment and persistence of CD4+ T cells and other immune cells at sites of HSV-2 reactivation as well as an increase in activated CD4+ T cells in the peripheral blood.
These findings prompted us to test the hypothesis that HSV-2 induces transcriptional changes in CD4+ T cells that promote HIV replication and/or reactivation.
We exposed primary CD4+ T cells or a latently HIV-infected T cell line to HSV-2 and, using RNA sequencing, identified significant transcriptional changes in the HSV-2 infected and bystander cells.
Significantly, the transcriptional changes were associated with an increase in HIV replication and reactivation.
One of the most significant responses we observed in both HSV-2 infected and bystander cells was an increase in expression of the long noncoding RNA, MALAT1. MALAT1 impacts the expression of multiple transcripts, including the HIV long terminal repeat (LTR), through interactions with the Polycomb Repressive Complex 2 (PRC2).
We knocked out MALAT1 in an HIV-latently infected cell line. We showed that HIV latency reversal was significantly reduced but not abolished when the knockout compared to the parental cells were infected with HSV-2.
Building on this foundation, we propose to further define MALAT1-mediated and identify MALAT1-independent mechanisms by which HSV-2 promotes HIV replication and reactivation.
We will take advantage of a longitudinal biorepository of peripheral blood mononuclear cells from people living with HIV and compare the responses to HSV-2 during periods of HIV viremia and viral suppression.
We will expose the cells to HSV-2, conduct single-cell RNA sequencing and proteomic and histone analyses, and determine how the cellular responses to HSV-2 enhance HIV replication and promote HIV latency reversal.
We will then focus on changes at the site of HSV-2 reactivation using genital skin biopsies collected at the time of an HSV outbreak (lesion and unaffected tissue) and after resolution in HIV-infected and uninfected individuals.
This will also allow us to assess interactions between different cell types, including epithelial cells, which are primary targets of HSV replication.
We will use cutting-edge, high-resolution spatial technologies to identify gene and protein expression in the tissue.
The results of these studies have great translational relevance and will identify new targets to promote or prevent HIV latency reversal that can ultimately lead to HIV eradication.
Our Trust Standards: Medical Advisory Committee