Can This Vaccine Conquer Herpes
Despite extensive research and hundreds of millions of infections, no government agency has yet approved an effective vaccine to prevent herpes simplex virus 2 (HSV-2) infection.
While current herpes vaccine development focuses on glycoproteins, recent studies suggest T cell responses against specific HSV-2 antigens are more prevalent in asymptomatic individuals.
Therefore, vaccine development should consider these differences in immune response to create a vaccine that can stimulate T-cell responses against these antigens.
In eClinicalMedicine, part of The Lancet, Laure F Pittet and colleagues published results from their phase 3 clinical trial, which was conducted across multiple countries in late 2023 to assess the impact of the very old tuberculosis Bacillus Calmette-Guérin (BCG) vaccine's implications for recurrent herpes labialis.
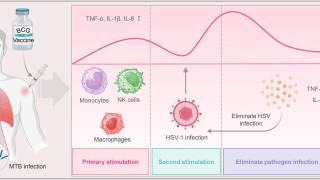
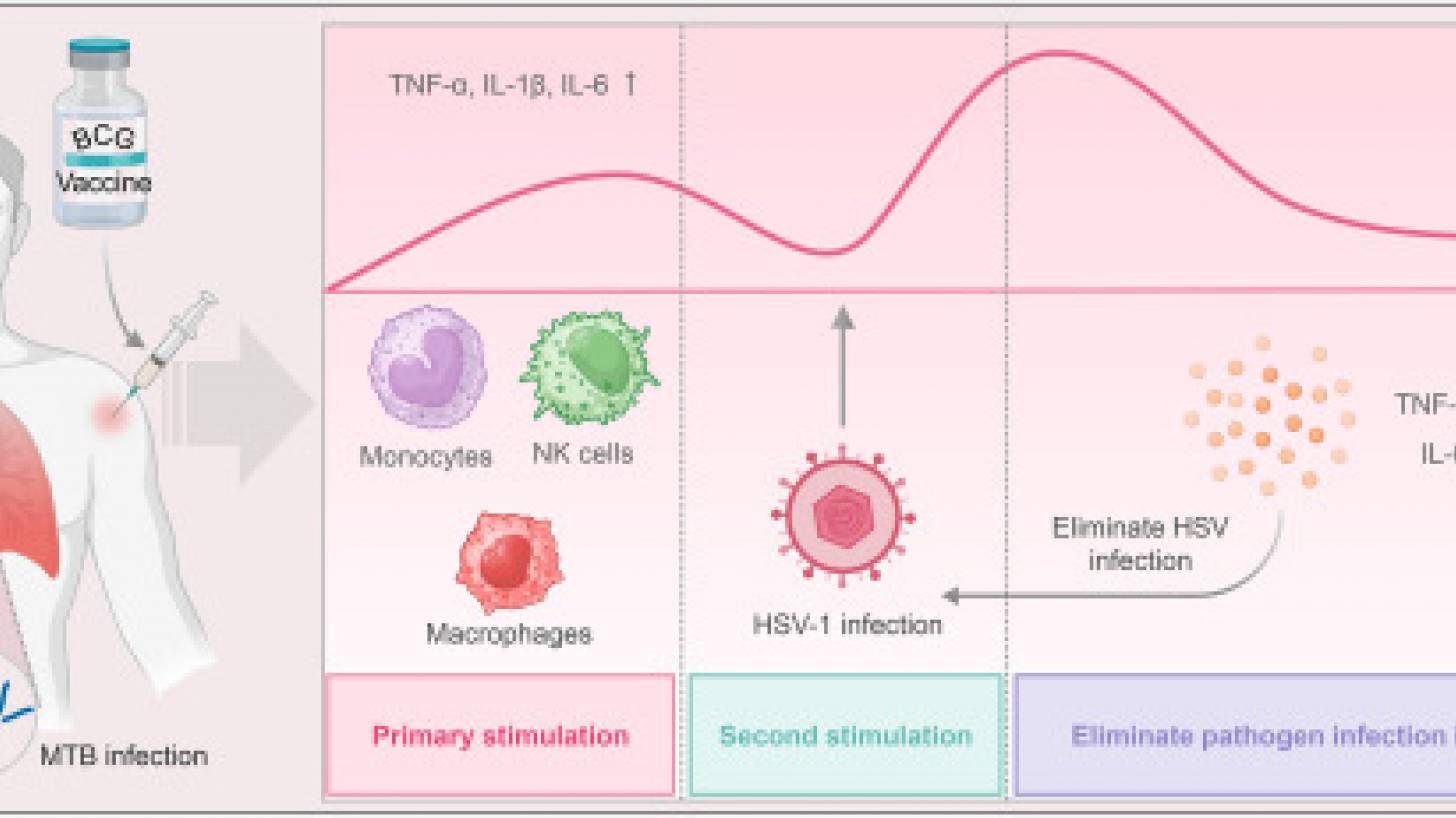
The results demonstrated that, over a one-year follow-up period, the BCG group experienced reduced duration, frequency, severity, and impact on quality of life-related to recurrent herpes labialis compared to the control group.
Furthermore, in the BCG-immunised individuals, the time to the first recurrence was extended by 1.55 months compared with the control group (p = 0.02), indicating the potential benefits of BCG vaccination for individuals with herpes labialis (p = 0.003).
A 2020 systematic review also demonstrated the benefits of BCG vaccination for 78% of adult patients with recurrent genital or herpes labialis, with 37% experiencing long-term remission and a reduction in outbreak frequency or severity by 41%.
Although these results differ from a 1985 observational clinical trial by J M Douglas and colleagues, which found that BCG vaccination did not significantly reduce the recurrence rate of genital herpes or prolong the time to the first recurrence, discrepancies may be attributed to variations in sample size, BCG vaccine types, dosage, administration methods, and the immunological status and genetic backgrounds of vaccine recipients.
A cautious interpretation of these clinical trial results is necessary, as the authors acknowledge various study limitations.
Currently, there is no universal BCG vaccination policy.
As of March 10, 2024, about 10 BCG vaccine versions are available worldwide.
In the U.S., the Centers for Disease Control and Prevention recommends the BCG vaccine for TB and bladder cancer patients.
Our Trust Standards: Medical Advisory Committee