Another mRNA Herpes Vaccine Candidate Launches

The race to produce a herpes virus vaccine heated up today with the announcement of a second mRNA-based clinical trial commencing.
Biopharmaceutical New Technologies (BioNTech) SE, a global leader in mRNA vaccines, announced that the first subject was dosed in a Phase 1 clinical research study with BNT163, a herpes simplex virus (HSV) vaccine candidate for the prevention of genital lesions caused by HSV-2 and potentially HSV-1.
The first-in-human trial evaluates the safety, tolerability, and immunogenicity of BNT163.
This mRNA vaccine encodes three HSV-2 glycoproteins intending to help prevent HSV cellular entry and spread, as well as counteract the immunosuppressive properties of HSVs.
Previously, Massachusetts-based Moderna Inc. announced it was developing the mRNA-1608 vaccine candidate in pre-human studies to reduce the burden of HSV lesions in May 2022.
Today's announcement is important since the U.S. Food and Drug Administration and agencies in Europe, Canada, India, and the U.K. have not Authorized or Approved any herpes prevention vaccine.
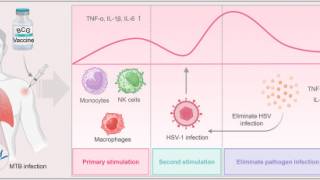
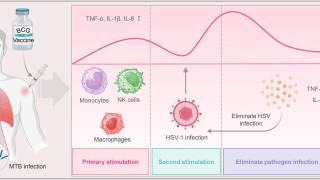
As neurotropic and neuroinvasive viruses, herpes viruses persist in the body by hiding from the immune system in the cell bodies of neurons where they reside lifelong and thus cannot be eradicated with current treatments.
"This program is part of our strategy to help address diseases with a high unmet medical need and of global health relevance by combining our new technologies, such as mRNA, and our expertise in immune engineering," said Prof. Özlem Türeci, M.D., Chief Medical Officer and Co-Founder of BioNTech, in a press release on December 21, 2022.
"BNT163 is based on three non-infectious mRNA-encoded HSV-2 glycoproteins."
"We aim to induce a broad immune response directed against multiple antigens of the virus and mobilizes various immune effectors to support virus neutralization and clearance."
In 2018, the University of Pennsylvania's Perelman School of Medicine (Penn) and BioNTech entered a research collaboration and license agreement to develop novel mRNA vaccine candidates for the prevention and treatment of various infectious diseases.
"My colleagues and I are proud to have contributed to the early development and preclinical testing of this exciting new mRNA vaccine candidate that may have the potential to prevent people from contracting the virus," commented Prof. Harvey M. Friedman, M.D., Professor of Infectious Diseases at Penn, who conducted preclinical and discovery science work on HSV and is Penn's principal investigator for the preclinical discovery and IND-enabling studies.
Herpes Simplex Virus-1 (HSV-1) and Herpes Simplex Virus-2 (HSV-2) cause two highly prevalent viral infections globally, says the World Health Organization.
Up to 95% of the global population is estimated to be infected by herpes, with most of the infections remaining asymptomatic.
Symptoms of herpes include painful blisters or ulcers that can recur over time.
- HSV-1 is mainly transmitted by oral contact and causes lesions around the mouth, but in some cases can also lead to genital infections and respective lesions.
- HSV-2 is a sexually transmitted disease that causes genital herpes. Both viruses are highly contagious and can also be transmitted during childbirth. Infections with HSV-2 further increase the risk of acquiring and transmitting HIV infections.
Located in Germany, Biopharmaceutical New Technologies is a next-generation immunotherapy company pioneering novel therapies for cancer and other serious diseases. The Company exploits a wide array of computational discovery and therapeutic drug platforms to develop novel biopharmaceuticals rapidly.
Additional herpes vaccine candidate development news is posted at PrecisionVaccinations.com/Herpes.
PrecisionVaccinations publishes fact-checked, research-based vaccine information manually curated for mobile readers.
Our Trust Standards: Medical Advisory Committee