U.S. Government Takes Herpes Vaccine Development Lead

Herpes simplex viruses (HSV) are widely known to cause viral infections such as blisters around the face, and genital herpes usually affects the genital and buttocks area.
Recent research suggests that HSV may also be linked to Alzheimer's and autoimmune diseases.
Unfortunately, there are no preventive vaccines currently available for this pervasive sexually transmitted disease (STD). Despite several herpes vaccine candidates undergoing clinical trials as of April 4, 2024, none had yet been approved by the U.S. FDA.
To address this shortcoming, the U.S. government issued a Notice of Special Interest (NOSI) NOT-AI-24-028 on March 27, 2024, intending to stimulate additional focus on developing diagnostics, therapeutics, and vaccines targeting HSV.
This NOSI, issued by the U.S. National Institute of Allergy and Infectious Diseases, focuses on furthering the development of new products for the prevention of HSV infection and improving the diagnosis and treatment of living with herpes.
It seeks applications that focus on the following:
- Accurate and convenient tests for diagnosing both symptomatic and asymptomatic herpes infection. This NOSI also calls for the development of diagnostic tests for CNS complications of HSV that do not rely on cerebrospinal fluid sampling.
- Novel antiviral molecules, especially those directed against a novel target.
- Therapeutic strategies for eliminating latent HSV. Further, it would support new technologies to cure HSV infection by reducing or removing latent HSV. This NOSI also calls for treatment strategies designed to minimize sequelae of HSV encephalitis.
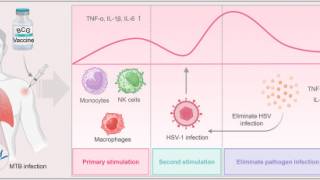
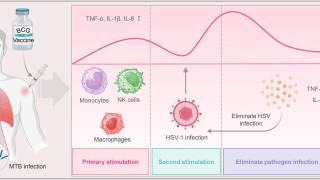
- Therapeutic and preventative vaccines. Previous vaccine candidates have focused on glycoprotein D (gD) as a protective antigen with limited results. Current research has identified other candidates that may provide protective benefits alone or combined with gD.
- Monoclonal antibodies for therapy or prevention of transmission. As an example, a multi-country research study is recruiting 332 healthy adult participants with recurrent genital herpes. Known as GSK3943104A, the GSK immunotherapy is forecasted to conclude in May 2026.
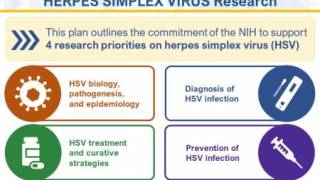
Furthermore, the NOSI supports the U.S. NIH Strategic Plan for HSV Research 2023-2028, which focuses on four strategic priorities: HSV virology basic research, better HSV diagnostics, strategies to address HSV treatment and cure, and research to prevent HSV infection.
The NIH confirmed that this notice (NOT-AI-24-028) applies to due dates on or after June 5, 2024, and subsequent receipt dates through April 5, 2024. Interested organizations should submit applications for this initiative using a specific notice of funding opportunity or any reissues of these announcements through the expiration date of this notice.
All instructions are in the SF424 Application Guide.
Our Trust Standards: Medical Advisory Committee