About Us
Precision Vaccinations Overview
Precision Vaccinations LLC (PVax) is an international publisher of trustworthy digital resources that empower people to make more informed vaccine decisions. Our research-based information is reviewed by doctors, nurses, and pharmacists. PVax's innovative publishing model—an enhanced Wiki for Vaccines—translates clinical information into a language people can understand.
PVax's goal is to enable more robust conversations between healthcare providers and patients to reduce the under-use, over-use, and misuse of vaccines.
'The old saying was... there are generally three sides to the truth.... yesterday's research, today's news, and the actual truth.'
'In the age of AI-produced content and biased search results, we believe vaccine science is not subjective, and trusted healthcare providers should verify vaccine information before advising patients.
'This philosophy empowers everyone to take action and live healthier lives.'
PVax Mission Statement
Vaccine information empowers people to live life to the fullest, and we believe vaccine information publishers must earn their readers' trust every day!
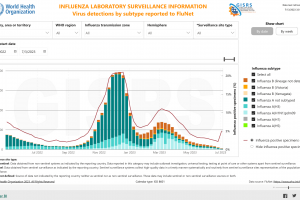
Virology
Over decades, the U.S. FDA's position on virology has evolved from preventing disease to empowering human immune systems to produce protection from disease. Since the Human Genome Project's success in 2000, people have gained awareness of their genetic makeup and ways to enhance their quality of life.
According to the U.S. CDC, almost 3.5 billion vaccine doses have been distributed in the United States over the past ten years. Research says that for every $1 spent on vaccines, $3 in indirect benefits are realized. In 2023, because of recent legislative changes, pharmacists administered more vaccines than any other healthcare provider.
The leaders of the FDA wrote in 2024 that the best way to counter the current large volume of vaccine misinformation is to dilute it with large amounts of truthful, accessible scientific evidence. On February 15, 2024, the FDA's Dr. Peter Marks informed the U.S. House Committee, ''Vaccines are one of the most highly effective public health interventions, responsible for saving millions of lives each year.' Marks, Director of the Center for Biologics Evaluation and Research, said no vaccine is 100% safe for all people.
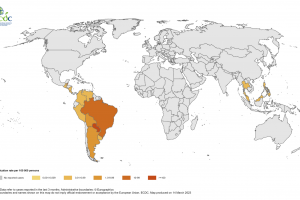
The CDC says during pretravel consultations, travel health providers must consider potential interactions between vaccines and medications. A study by S. Steinlauf et al. identified potential drug–drug interactions with travel-related medications in 45% of travelers taking medications for chronic conditions; 3.5% of these interactions were potentially serious. This link helps explain Drug / Vaccine interactions, Informed Consent, and U.S. FDA Package Inserts.
Vaccine Development's Future
The promise of personalized vaccines, led by the Precision Vaccines Program, which is discovering and developing the next generation of vaccines tailored to sub-populations, may disrupt the one-size-fits-all vaccination model. From a size of market perspective, the global clinical trials market was evaluated at $51.17 billion in 2023 and is expected to attain around $80 billion by 2032, growing at a CAGR of 5.6%. The vaccine sub-market reached $102.9 billion in 2023 and is forecasted to expand by 47% to reach $35.1 billion by 2030. A report published in November 2023 highlighted 1,600 treatments and vaccines in clinical development just for cancer. And the global travel vaccines market is estimated to increase at a CAGR of 9.9% through 2028. In the past ten years, companies with infectious disease vaccine programs received 3.4% of the total ($6.5 billion) venture capital raised for biopharmaceutical companies. In 2023, clinical trials began rapidly integrating artificial intelligence and Machine Learning models to rapidly advance vaccine development.
Vaccine Publishing
The World Health Organization has confirmed vaccine misinformation is a significant threat to global health that could reverse decades of progress in reducing disease. Editorial Standards have been refined over 20 years and are committed to publishing accurate information. We stand by the Editorial Independence as our Medical Advisory Team works closely with pharmacists, doctors, and nurses across various specialty areas to ensure PVax's published content is accurate, actionable, and understandable.
Visit this PVax page to access PVax's complete publishing disclosures.
Policies
Various PVax policies are listed on this webpage.
Business Overview
PVax ownership and revenues are discussed at this link.
Comments and Concerns
We believe that public feedback is not a one-way street. Instead, we are committed to engaging with you and acting based on your suggestions and other feedback. We are committed to providing greater transparency about how news is produced and offering regular contact and interaction points. We will respond to your request ASAP. Would you mind sending an email to [email protected].
Thank you,
Karen Hackett, Founder & President