$504,000 Funds Herpes Vaccine Research

While there are no approved herpes vaccines available in 2023, the U.S. government recently funded research being conducted at the University of Pittsburgh School of Medicine.
The National Institute of Allergy and Infectious Diseases (NIAID) confirmed on November 1, 2023, that it had awarded $504,170 to a research project led by Neal A. DeLuca.
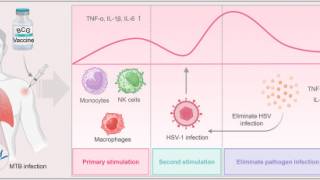
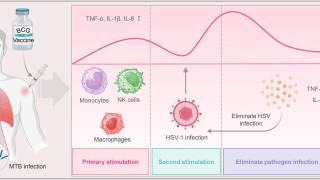
The ongoing theme of this innovative program is to study the virus-cell interactions that affect Herpes simplex virus (HSV) gene expression.
Detailed knowledge of how HSV genes are transcribed and the virus-cell interactions that contribute to their regulated expression may allow for strategies to block activated transcription and hence virus multiplication, wrote the NIAID.
Progress during the last funding period has provided new insights into how the genes of HSV are transcribed in a regulated cascade during the course of viral infection.
The studies also showed how viral processes and molecules can interact with and possibly regulate cellular transcription.
The HSV genome during productive infection is a much more dynamic structure than classic cellular chromatin. The viral nucleoprotein allows for rapid progression through the infectious cycle so that by 3hpi (hours post infection), all the events that must occur on the viral genome to produce a new virus can be observed.
This includes events that contribute to the shutoff of host cell transcription.
This renewal focuses on virus-cell interactions occurring on the cellular (aim 1) and viral (aim 2) genomes, as well as the virus's utilization of a cellular complex that is crucial for all pol II transcription, the mediator (aim 3).
We have shown that the significant viral regulatory protein, ICP4, binds to promoter/start-site regions of specific cellular genes early in infection as a function of the cell's epigenetic state, wrote these researchers.
The first aim is to investigate the mechanism of interaction between ICP4 and the cellular genome, determine how ICP4 affects its expression, and explore the possibility that it functions differently in different cell types.
The second will focus on the structure of the viral genome and how viral proteins, including ICP4, affect the accessibility of the genome and, hence, viral transcription. During the last funding period, we showed that ICP4 interacts with and recruits a specific form of the mediator to the viral genome.
In the third aim, we will further explore the ICP4-mediator interaction and test a model for recruiting mediators by ICP4 and its utilization for the activated and regulated transcription of viral genes.
To undertake these studies, we have developed and refined a set of proteomics and genomics tools to specifically probe the dynamic state of viral genomes and the viral and cellular proteins that associate with them. These tools combined with virus genetics allow us to elucidate the mechanistic underpinnings of processes occurring on the viral genome, including transcription, concluded these researchers.
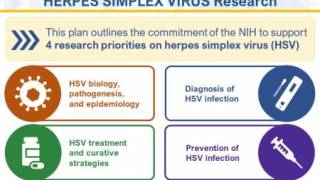
In September 2023, the U.S. NIH published the Strategic Plan for Herpes Simplex Virus Research for 2023-2028 to advance herpes vaccine research.
Herpes simplex virus 1 and 2 are among the most common viral infections in the U.S., with up to 80% of people between the ages of 14 and 49 infected with HSV-1 and more than 10% infected with HSV-2.
Infection with HSV can result in a diverse range of symptoms and pathology that can lead to lifelong complications.
Additionally, the World Health Organization (WHO), the U.S. National Instuties of Health, and global partners launched STI Watch, a digital portal containing updated information on vaccine development status.
On July 24, 2023, the WHO announced its latest guidance (4th edition) on sexually transmitted infections, including point-of-care tests and target vaccine profiles.
Our Trust Standards: Medical Advisory Committee