Second Malaria Vaccine Authorized in Africa
The University of Oxford today announced the R21/Matrix-MTM malaria vaccine had been licensed for use in the Republic of Ghana by the local Food and Drugs Authority.
The R21/Matrix-M™ malaria vaccine was developed by the University of Oxford, leveraging an adjuvant technology from Novavax, and manufactured and scaled up by Serum Institute of India Pvt Ltd (SIIPL).
SIIPL has already established manufacturing capacities of more than 200 million vaccine doses annually.
As of April 13, 2023, this is the first regulatory clearance for the R21/Matrix-M malaria vaccine for use in any country for children aged 5 to 36 months.
Notably, the R21/Matrix-M vaccine has previously demonstrated high levels of efficacy and safety in Phase II trials, including amongst children who received a booster dose of R21/Matrix-M at one year following a primary three-dose regime.
The ongoing phase III trial in Burkina Faso, Kenya, Mali, and Tanzania has enrolled 4,800 children, with results expected later in 2023.
Prof. Adrian Hill, Chief investigator R21/Matrix-M program and Director of the University of Oxford's Jenner Institute at the Nuffield Department of Medicine commented in a related press release, "This marks a culmination of 30 years of malaria vaccine research at Oxford with the design and provision of a high efficacy vaccine that can be supplied at adequate scale to the countries who need it most."
The R21/Matrix-M malaria vaccine is a low-dose vaccine that can be manufactured at a modest cost, enabling as many as hundreds of millions of doses to be supplied to African countries suffering a significant malaria burden.
The vaccine contains Novavax's Matrix-M, a saponin-based adjuvant that enhances the immune system response, making it more potent and durable. The Matrix-M adjuvant stimulates the entry of antigen-presenting cells at the injection site and enhances antigen presentation in local lymph nodes.
In response to this announcement, Javier Guzman, Senior Policy Fellow and Director of Global Health Policy at the Center for Global Development, commented in an email to Vax-Before-Travel, "It is very exciting that the regulatory agency in Ghana has decided to license the R21 vaccine after reviewing data from all clinical trials."
"Ghana has a stable, well-functioning, and integrated regulatory system, according to an external benchmarking conducted by the World Health Organization in 2020.
"However, this does not mean that donors or international vaccine procurers such as Gavi or UNICEF will fund the vaccine."
"These agencies still require that the vaccine is considered safe, effective, and quality assured by the World Health Organization (WHO) prequalification program."
"Also, it is still uncertain if R21 is good value for money, especially compared to other cost-effective malaria interventions that have not been fully deployed across endemic countries, such as insecticide-treated nets or indoor residual spraying."
"In other words, while the vaccine might be heralded as a huge win in the fight against malaria, it is no silver bullet, and there are important points to consider before the R21 vaccine is rolled out for wider use."
The WHO previously Listed the Mosquirix™ (RTS,S) malaria vaccine for use in Ghana and other African countries.
Furthermore, malaria vaccine candidates are conducting clinical trials as of April 13, 2023.
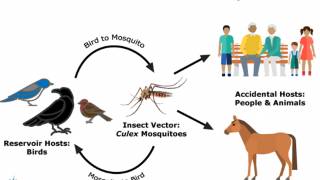
Malaria is a disease caused by a parasite transmitted to people by an infected mosquito's bite. It is always a severe disease and may be a deadly illness.
And the U.S. Centers for Disease Control and Prevention (CDC) says millions of U.S. residents travel to countries where malaria is present. About 2,000 cases of malaria are diagnosed in the U.S. annually, mostly in returned travelers.
The CDC has issued various malaria outbreak alerts for 88 malaria-endemic countries to alert travelers of this health risk.
In the Region of the Americas, Brazil and Venezuela reported the most malaria cases in 2022.
Our Trust Standards: Medical Advisory Committee