Malaria Vaccination Quickly Loses Protective Effect
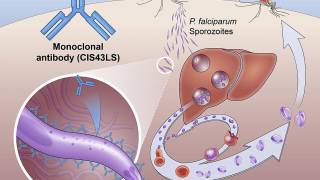
Scientists from the German Cancer Research Center (DKFZ) recently announced they studied how the human immune response after immunization with the malaria pathogen Plasmodium falciparum (Pf).
Their goal was to find out against which protein components the T helper cells induced in this way are directed.
Published by the journal Science Immunology on June 10, 2022, this study revealed T helper cells reacted exclusively to the protein sequence of the vaccine strain and showed hardly any cross-reactivity with the naturally occurring pathogen variants.
‘This could explain why natural infections, to which people in malaria-endemic areas are constantly exposed, offer little protection against new diseases with other strains and why the effect of the malaria vaccination available to date lasts only a short time.’
‘These data suggest that the high parasite diversity in endemic areas limits boosting the vaccine-induced T cell response by natural infections.’
‘Our findings may guide the further design of the Pf circumsporozoite protein (PfCSP) based malaria vaccines able to induce potent T helper cell responses for broad, long-lasting antibody responses.’
The vast majority of fatal cases of malaria are caused by the pathogen Plasmodium falciparum. Unfortunately, to date, there is only one approved vaccine against this single-celled organism, and its efficacy, which is already relatively low, does not last long.
The vaccine is directed against CSP, the quantitatively dominant protein on the surface of the "sporozoites."
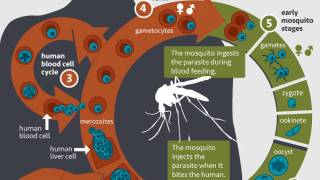
Sporozoites are the stage of the malaria pathogen which is transmitted with the mosquito bite and enters human blood.
"To improve the vaccine, we need to understand which immunization induces protective antibodies. But the production of such antibodies depends largely on help from the so-called follicular T helper cells," says Hedda Wardemann of the German Cancer Research Center.
"They ensure that B cells transform into antibody-producing plasma cells and memory B cells."
To study the T helper cell response against CSP in detail, the team led by DKFZ immunologist Wardemann examined the blood of volunteers infected with killed P. falciparum sporozoites from the vaccine strain.
The researchers analyzed the induced Plasmodium-specific follicular T helper cells at the single-cell level. In particular, they focused their investigation on which sequences of CSP are recognized by the receptors of the T helper cells.
The analyses revealed that the T-cell receptors mainly targeted amino acids 311 to 333 of the CSP.
But another observation stunned the researchers: virtually no cross-reactivity between the individual T-cell clones.
"The receptors highly specifically bind only the CSP epitopes of the vaccine strain used. Therefore, even deviations of only a single amino acid component were not tolerated in some cases," Wardemann explained.
The immunologist points out that in the natural population of P. falciparum, sequence polymorphisms occur to a high degree in this region of the CSP.
"The specificity of the T-cell clones prevents the constantly recurring natural infections with the pathogen from acting as a natural 'booster.' This could explain why the protective effect of the malaria vaccine wears off so quickly," Wardemann added.
The researcher recommends that further vaccine development should test whether inducing a broader spectrum of T helper cells could generate longer-lasting immune protection.
In October 2021, the World Health Organization (WHO) recommended GSK’s Mosquirix™ RTS,S/AS01 (RTS,S) malaria vaccine for children at risk in sub-Saharan Africa and other regions. This is a recombinant malaria vaccine with the P. falciparum circumsporozoite protein (CSP) from the pre-erythrocytic stage.
Mosquirix also helps protect against liver infection with the hepatitis B virus but should not be used only for this purpose. This is the only malaria vaccine approved for use by the European Medicine Agency but is not authorized by the U.S. FDA.
In April 2022, PATH received a grant of close to $5 million to enable the expanded introduction of the Mosquirix malaria vaccine in areas of Ghana, Kenya, and Malawi.
“WHO welcomes this new funding that will ensure more children at risk can benefit from this life-saving vaccine,” commented Mary Hamel, M.D., WHO Team Lead for Malaria Vaccines and the WHO Lead for the MVIP, in a media statement.
“Malaria remains a major cause of child deaths in Africa, and the malaria vaccine has shown it can substantially reduce life-threatening severe malaria and child deaths.”
“However, the vaccine can only reach its full potential if it reaches children who need it.”
About 2,000 cases of malaria are diagnosed in the USA annually, mostly in returned travelers, says the U.S. CDC.
‘‘All travelers to countries where malaria is present may be at risk for infection.’
PrecisionVaccinations publishes fact-checked, research-based vaccine news curated for mobile readership.
Our Trust Standards: Medical Advisory Committee