Pregnant Women Should Avoid Malaria in Florida

When the Florida Department of Health (DOH) and the Centers for Disease Control and Prevention (CDC) confirmed a sporadic local case of Plasmodium vivax malaria in Sarasota and Manatee Counties in May 2023, most Floridians were surprised as most malaria cases diagnosed in the United States are imported.
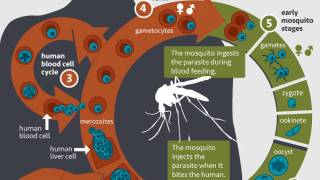
The DOH and CDC stated on June 2, 2023, locally acquired mosquito-transmitted malaria cases could occur, as Anopheles mosquitoes are found throughout the U.S.
For example, 8 cases of locally acquired P. vivax malaria were identified in Palm Beach County, FL, in 2003.
However, the risk of locally acquired malaria is extremely low in the U.S., as is the morbidity and mortality for most people.
But, due to the risk of progression to severe disease, pregnant women and their unborn children have a significantly greater health risk from malaria infections, wrote the CDC in February 2023.
Pregnant women are three times more likely to develop severe disease than non-pregnant women who acquire malaria in the same geographic area.
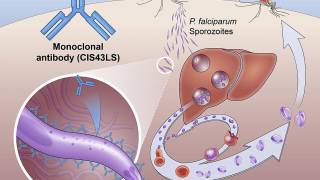
In addition, malaria parasites sequester and replicate in the placenta.
Malaria infection during pregnancy can lead to miscarriage, premature delivery, low birth weight, congenital infection, and/or perinatal death.
While the mechanism is poorly understood, pregnant women have a reduced immune response to malaria treatment and, therefore, less effectively clear malaria infections.
Pregnant women of all gestational ages diagnosed with uncomplicated malaria acquired in areas with chloroquine-resistant P. falciparum can be treated with artemether-lumefantrine, mefloquine, or a combination of quinine sulfate and clindamycin.
Quinine treatment should continue for seven days for P. falciparum infections acquired in Southeast Asia and three days for infections acquired elsewhere. And clindamycin treatment should continue for seven days regardless of where the infection was acquired.
Prompt treatment with chloroquine or hydroxychloroquine for pregnant women diagnosed with uncomplicated malaria caused by P. malariae, P. ovale, chloroquine-sensitive P. vivax, or chloroquine-sensitive P. falciparum infection is recommended.
For chloroquine-resistant P. vivax infections, artemether-lumefantrine, quinine plus clindamycin, or mefloquine should be given instead.
For P. vivax or P. ovale infections, primaquine phosphate and tafenoquine for radical treatment of hypnozoites should not be given during pregnancy, says the CDC.
Pregnant patients with P. vivax or P. ovale infections should be maintained on chloroquine chemoprophylaxis for their pregnancy.
The chemoprophylactic dose of chloroquine phosphate is 300 mg base (500 mg salt) orally once weekly.
After delivery, for new mothers with regular G6PD activity infected with P. vivax or P. ovale infections, subsequent treatment with primaquine phosphate or tafenoquine as described above is needed but will depend on breastfeeding.
If not breastfeeding, either drug can be used depending on the regimen used to treat the acute malaria episode.
For breastfeeding women, infants should be tested for G6PD deficiency, and if found to have normal activity, oral primaquine phosphate can be given to the mother.
Tafenoquine is not recommended during breastfeeding, says the CDC.
Mothers following delivery who cannot take primaquine or tafenoquine should be on weekly chloroquine chemoprophylaxis for one year after the acute malaria episode.
And, Artesunate for Injection™ was FDA approved in May 2020, indicated for infants, children, adults, and pregnant women with severe malaria or those unable to tolerate oral antimalarials.
In addition, clinicians should hospitalize patients with P. falciparum infection to monitor clinical response and check parasite density every 12–24 hours. Once clinical presentation improves and a decrease in parasite density becomes apparent, treating clinicians can consider outpatient completion of treatment.
Emergency treatment consultation advice is available for health care providers in Florida and elsewhere through the CDC Malaria Hotline (770-488-7788 or 855-856-4713 toll-free) from 9:00 a.m. to 5:00 p.m. ET.
Furthermore, while two malaria vaccines have been approved for use in Africa, they are unavailable in the U.S. as of June 13, 2023.
Our Trust Standards: Medical Advisory Committee