Malaria Vaccine Supply Significantly Increases
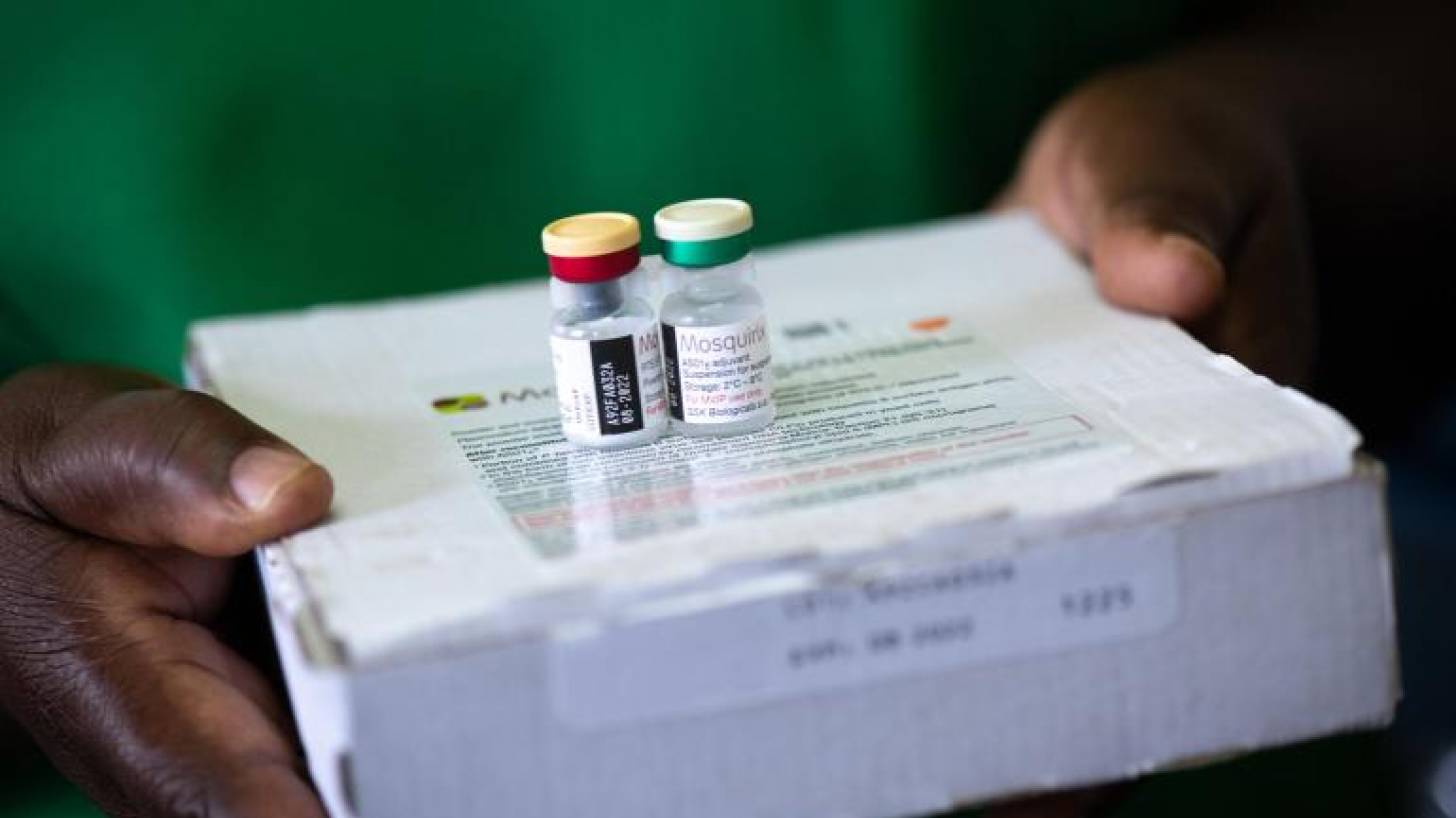
UNICEF recently announced it had awarded a contract with a value of up to USD 170 million that will lead to 18 million doses of Mosquirix™ RTS,S/AS01 (RTS,S) being available over the next three years, potentially saving thousands of lives every year.
GSK's Mosquirix is a recombinant malaria vaccine that is the first and only malaria vaccine shown in pivotal long-term clinical trials to reduce malaria in children significantly.
"This vaccine rollout gives a clear message to malaria vaccine developers to continue their work because malaria vaccines are needed and wanted," said Etleva Kadilli, Director of UNICEF's Supply Division, in a press release issued on August 16, 2022.
"We hope this is just the beginning."
"Continued innovation is needed to develop new and next-generation vaccines to increase available supply and enable a healthier vaccine market."
"This is a giant step forward in our collective efforts to save children's lives and reduce the burden of malaria as part of wider malaria prevention and control programs."
UNICEF procures over two billion vaccines annually for routine immunization and outbreak response on behalf of nearly 100 countries.
According to WHO data, more than 30 countries have moderate to high malaria transmission areas.
Therefore, the vaccine could potentially add protection to over 25 million children each year.
Demand for the Mosquirix malaria vaccine is expected to be high among affected countries.
As with any new vaccine, supply will be limited at first and will increase over time as manufacturing capacity ramps up to the level required.
Plans are already underway to boost production, including through technology transfer, so that every child at risk will one day have the opportunity to be immunized against this killer disease.
"We must not lose sight of the need to accelerate access to this and future malaria vaccines and to make the necessary investments in malaria control and immunization services, as well as in research and development," commented Dr. Ashley Birkett, Global Head of Malaria Vaccines & Biologics at PATH.
"Effective malaria and immunization programs are both key to the successful delivery of a malaria vaccine and contribute to stronger health systems overall."
The RTS,S malaria vaccine results from 35 years of research and development.
In 2019, pilot routine vaccine use was launched in Ghana, Kenya, and Malawi as part of the Malaria Vaccine Implementation Programme coordinated by WHO.
The experience and evidence generated by the pilots informed WHO's recommendation in October 2021 for the widespread use of the first malaria vaccine in countries with moderate to high P. falciparum malaria transmission.
Soon after, in December 2021, Gavi, the Vaccine Alliance's decision to provide funding for malaria vaccine programs in eligible countries opened the pathway for a broader rollout of the vaccine.
Thomas Breuer, Chief Global Health Officer, GSK, said in a press release on December 2, 2021, "Our malaria vaccine is a new and important tool that is urgently needed given rising numbers of malaria cases in African children."
"The vaccine has the potential to have a significant impact on the burden of malaria in Sub-Saharan Africa."
Mosquirix does not provide complete protection against malaria caused by P. falciparum, says the U.S. CDC.
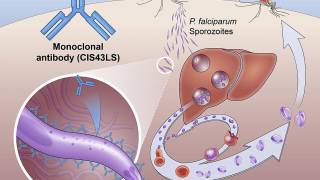
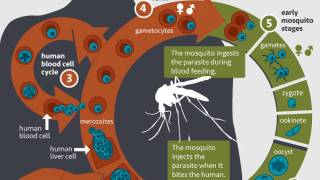
The malaria vaccine is designed to prevent the parasite from infecting the liver, entering the bloodstream, infecting red blood cells, and leading to disease symptoms.
In addition, because of the vaccine's composition, it also protects against the hepatitis B virus.
Malaria is a mosquito-borne disease caused by a parasite.
Left untreated, they may develop severe complications and die. In 2020 an estimated 241 million cases of malaria occurred worldwide, and 627,000 people died, mostly children in sub-Saharan, says the CDC.
About 2,000 cases of malaria are diagnosed in the United States annually, mostly in returned travelers.
Travelers to sub-Saharan Africa have the most significant risk of both getting malaria and dying from their infection.
The risk for a traveler contracting malaria differs substantially from region to region and from traveler to traveler, even within a single country, based upon travelers' behaviors and circumstances, says the CDC.
The CDC's Malaria Risk Assessment for Travelers is available on this weblink.
As of August 16, 2022, the U.S. FDA has not approved a malaria vaccine.
Vax-Before-Travel publishes fact-checked, research-based travel vaccine news curated for international travelers.
Our Trust Standards: Medical Advisory Committee