Malaria Infections Prevented with Monoclonal Antibody
A recent U.S. National Institutes of Health (NIH) clinical trial found for the first time one dose of an antibody drug safely protected healthy, non-pregnant adults from malaria infection during an intense six-month malaria season in Africa.
The antibody was up to 88.2% effective at preventing infection over 24 weeks, demonstrating that a monoclonal antibody can prevent malaria infection in an endemic region.
The NIH says there is an urgent need for new, fast-acting, infrequently dosed interventions that safely protect against malaria infection.
"We need to expand the arsenal of available interventions to prevent malaria infection and accelerate efforts to eliminate the disease," said Anthony S. Fauci, M.D., director of the National Institute of Allergy and Infectious Diseases (NIAID), part of NIH, in a press release on October 31, 2022.
"These study results suggest that a monoclonal antibody could potentially complement other measures to protect travelers and vulnerable groups such as infants, children, and pregnant women from seasonal malaria and help eliminate malaria from defined geographical areas."
An estimated 241 million cases of malaria occurred worldwide in 2020, according to the World Health Organization (WHO), resulting in an estimated 627,000 deaths, mostly in children in sub-Saharan Africa.
These cases included more than 11 million pregnant women in Africa, resulting in an estimated 819,000 newborns with low birth weight and thus at increased risk for illness and death.
The only malaria vaccine currently recommended by WHO, called Mosquirix™ RTS,S/AS01 (RTS,S), provides partial protection against clinical malaria during the early years of life when given to children aged 5 to 17 months in four doses over 20 months.
Mosquirix is available in Malawi, Kenya, and Ghana and received WHO PreQualification on September 6, 2022.
Other drugs consisting of small chemical compounds that effectively prevent malaria infection are also available for infants, young children, and travelers.
The requirement for frequent dosing of these drugs can limit adherence, however, and the emergence of drug resistance may also limit their usefulness.
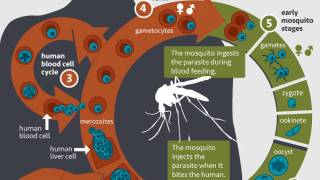
Malaria is caused by Plasmodium parasites transmitted to people through the bite of an infected mosquito.
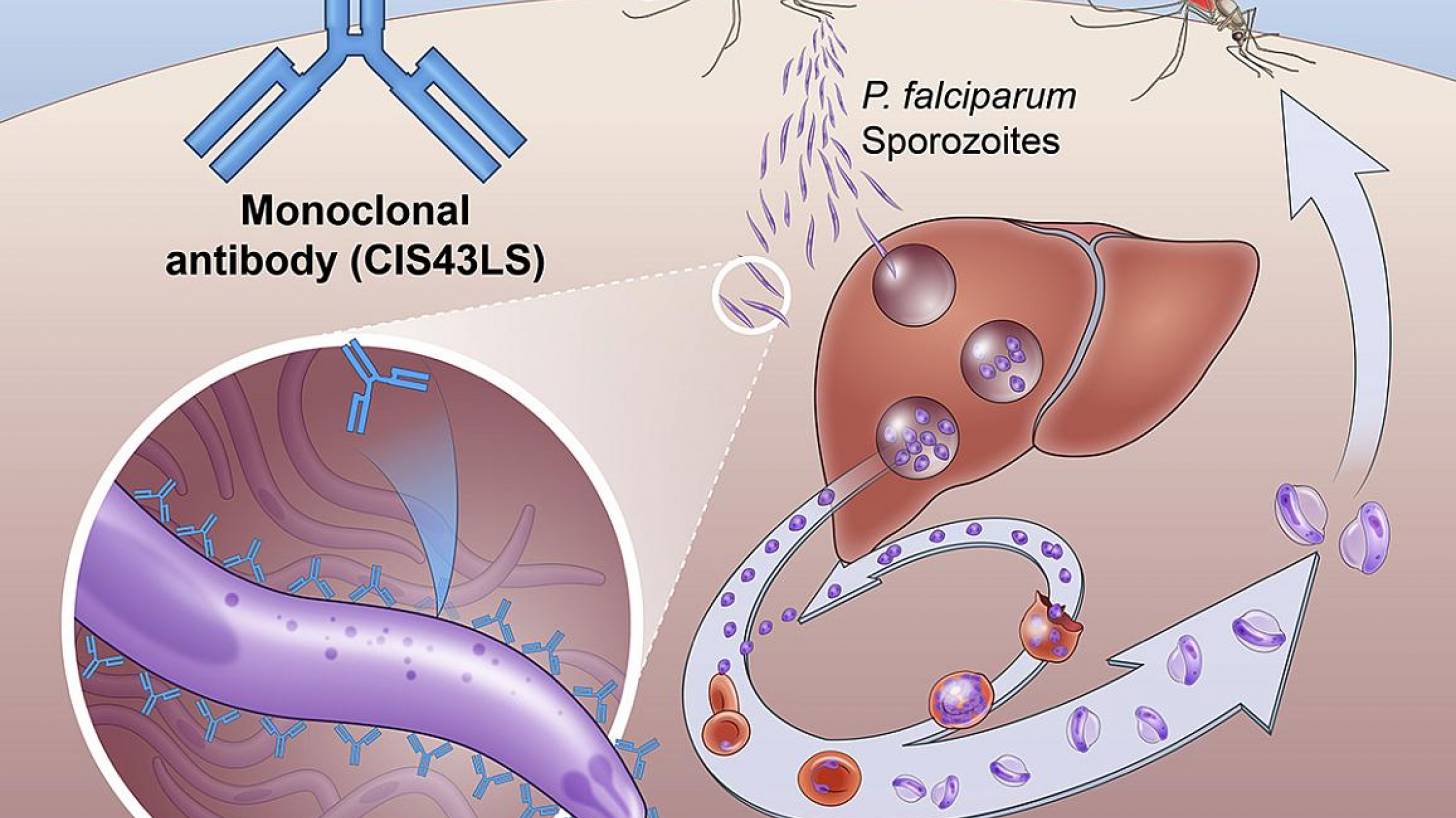
The mosquito injects the parasites in sporozoites into the skin and bloodstream.
These travel to the liver, where they mature and multiply.
Then the mature parasite spreads throughout the body via the bloodstream to cause illness. P. falciparum is the Plasmodium species most likely to result in severe malaria infections, which, if not promptly treated, may lead to death.
The Phase 2 NIAID-USTTB trial evaluated the safety and efficacy of a one-time, intravenous infusion of a monoclonal antibody called CIS43LS. This antibody was previously shown to neutralize the sporozoites of P. falciparum in the skin and blood before they could infect liver cells.
The first part of the trial assessed the safety of three different doses of CIS43LS — 5 milligrams per kilogram of body weight, 10 mg/kg, and 40 mg/kg — administered by intravenous infusion in 18 study participants, with six participants per dose level.
The study team followed these participants for 24 weeks and found the antibody infusions were safe and well-tolerated.
The trial's second part assessed the efficacy of two different doses of CIS43LS compared to a placebo.
Three hundred and thirty participants were randomly assigned to receive either 10 mg/kg of the antibody, 40 mg/kg, or placebo by intravenous infusion.
No one knew who was assigned to which group until the end of the trial. The study team followed these individuals for 24 weeks, testing their blood for P. falciparum weekly for the first 28 days and every two weeks after that.
Any participant who developed symptomatic malaria during the trial received standard treatment from the study team.
The investigators analyzed the efficacy of CIS43LS in two ways.
Based on the time to first P. falciparum infection over the 24-week study period, the high dose (40 mg/kg) of CIS43LS was 88.2% effective at preventing infection, and the lower dose (10 mg/kg) was 75% effective.
An analysis of the proportion of participants infected with P. falciparum at any time over the 24-week study period found the high dose was 76.7% at preventing infection and the lower dose was 54.2% effective.
"We hope monoclonal antibodies will transform malaria prevention in endemic regions," commented these researchers.
Dr. Seder and colleagues have developed a second antimalarial monoclonal antibody, L9LS, that is much more potent than CIS43LS and, therefore, can be administered in a smaller dose as an injection under the skin (subcutaneously) rather than by intravenous infusion.
An early-phase NIAID trial of L9LS in the United States found that the antibody was safe and prevented malaria infection for 21 days in 15 out of 17 healthy adults exposed to P. falciparum in a carefully controlled setting.
Two larger, NIAID-sponsored Phase 2 trials assessing the safety and efficacy of L9LS in infants, children, and adults are underway in Mali and Kenya.
Additional information about the Phase 2 trial of CIS43LS is available at ClinicalTrials.gov under study identifier NCT04329104.
These findings were published in The New England Journal of Medicine and presented at the American Society of Tropical Medicine & Hygiene 2022 Annual Meeting in Seattle.
The NIAID conducts and supports research—at NIH, throughout the U.S., and worldwide—to study the causes of infectious and immune-mediated diseases and to develop better means of preventing, diagnosing, and treating these illnesses.
For more information about NIH and its programs, visit www.nih.gov.
Certified travel pharmacies in the U.S. offer malaria advice and related supplies.
"The currently available medications used for malaria prophylaxis depend on patient adherence to work, so there is a clear benefit to the international traveler for a monoclonal antibody that protects for 24 weeks, said Crockett Tidwell RPh, CDCES.
"If this product follows suit to monoclonal antibodies that are currently available for other disease states, the downside of more complicated administration, extended monitoring after the administration, and high cost could make its use more limited," added Tidwell, the Clinical Services Manager, United Pharmacy, in Lubbock, Texas.
Additional malaria prevention and treatment news are posted at Vax-Before-Travel.com/Malaria.
Vax-Before-Travel publishes fact-checked, research-based travel news manually curated for mobile readers.
Our Trust Standards: Medical Advisory Committee