Malaria Vaccine Chemoprevention Significantly Reduces Deaths

In light of the global expansion of malaria into Central America, Florida, and Texas in 2023, the results of a landmark study confirm the benefits of combining the RTS,S/AS01E (RTS,S) (Mosquirix™) malaria vaccine with antimalarial drugs.
Published in The Lancet Infectious Diseases on August 18, 2023, the vaccine-drug combination reduced clinical malaria episodes, including cases of severe malaria, and deaths from malaria in young children by nearly two-thirds compared with either RTS,S vaccination, or seasonal malaria chemoprevention (SMC) alone.
This Phase 3 study also confirmed that the efficacy of RTS,S in preventing malaria in highly seasonal settings was similar, or "non-inferior," to that of SMC.
Coordinated by the London School of Hygiene & Tropical Medicine (LSHTM) with partners, the study's findings from five years of follow-up of 5,000 children are consistent with those from the first three years, published in 2021.
Those findings contributed to the World Health Organization's (WHO) decision that year to recommend the RTS,S vaccine for use in settings of moderate-to-high malaria transmission, including its use in areas with highly seasonal malaria or areas of perennial malaria transmission with seasonal peaks.
These new findings confirm the potential of seasonal vaccination to provide a high level of protection in young children over the first five years of life when this protection is needed.
LSHTM's Professor Brian Greenwood, MD, a member of the research team, stated in a press release on August 22, 2023, "In addition to the study's findings—which by themselves are remarkable—we can say that children who received the RTS,S-drug combination and also used bednets likely had greater than 90% protection against malaria episodes during the study."
"This points to the importance of ensuring access to multiple malaria prevention tools for reducing the tremendous burden of malaria disease and death in these highly seasonal settings."
Additionally, children in the study who received SMC and/or the vaccine are being followed for another two years.
This follow-up will help to determine how long protection lasts and whether the high level of protection against malaria provided by the combination of SMC and seasonal RTS,S vaccination impaired the acquisition of naturally acquired immunity among the children in the study by reducing the number of infections they received in early life.
Currently, SMC is not given to children above the age of 5 in most countries where it is deployed.
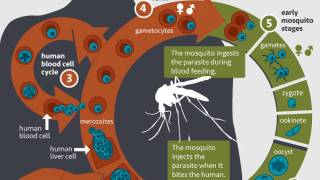
SMC, which involves giving antimalarial drugs sulfadoxine-pyrimethamine and amodiaquine to young children four or five times during the rainy season when malaria transmission peaks, is highly effective in preventing malaria and was recommended by WHO in 2012 for use in areas with highly seasonal transmission.
According to the World Health Organization Report, the global number of malaria cases reached about 240 million, with over 600,000 related fatalities in 2021.
To forewarn international travelers of this health risk, the U.S. Centers for Disease Control and Prevention has issued various malaria outbreak alerts for countries, including Costa Rica.
Malaria vaccines are approved for use in Africa but not the U.S. in 2023.
Our Trust Standards: Medical Advisory Committee