More Malaria Vaccines Coming in 2024
Gavi, the Vaccine Alliance, welcomed today's announcement, which can help reshape the global fight to reduce malaria outbreaks.
Following the joint session of the World Health Organization (WHO) Strategic Advisory Group of Experts on Immunization (SAGE) and the Malaria Policy Advisory Group, the WHO announced today that it has recommended a second malaria vaccine to prevent malaria in children in outbreak areas.
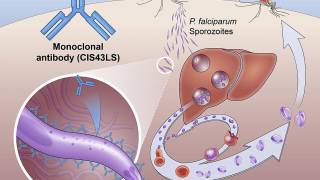
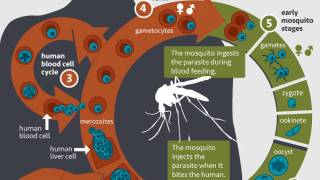
The R21/Matrix-M™ Malaria Vaccine is indicated to prevent Plasmodium falciparum malaria, a mosquito-borne disease.
Today's announcement means there are now two WHO-recommended vaccines that can help meet the high and increasing demand from malaria-endemic regions of the world.
"The joint recommendation of the R21/Matrix-M vaccine represents another major step towards our goal of creating a malaria-free life for every child," commented David Marlow, CEO of Gavi, in an October 2, 2023 press release.
"This vaccine and the existing RTS,S/AS01e (Mosquirix™) vaccine will complement existing malaria interventions. Once it receives WHO prequalification, it will play a key role in meeting the high demand we are seeing in (malaria) endemic countries."
Today's announcement emphasizes the critical role these two vaccines and other interventions will play as the malaria community aims to advance its goals over the next decade. Alliance partners are already in conversation with countries about the potential rollout of a second vaccine as soon as 2024, wrote Gavi.
As of October 2, 2023, these malaria vaccines are unavailable in the United States.
The R21 vaccine still awaits WHO prequalification, a precondition to the global rollout. When R21 is prequalified, it would greatly ease supply issues and help us begin to meet the high demand.
During 2023, Ghana and Nigeria issued authorizations for R21.
And SII Chief Executive informed Reuters in April 2023 that he is producing 20 million doses "at-risk."
Once a vaccine is prequalified, it can then be offered through Gavi programs.
In 2022, once the RTS,S/AS01 received WHO prequalification, Gavi opened a funding window for applications from countries that wished to roll it out through Gavi programs and with Gavi funding.
In 2023, 18 million doses of RTS,S were allocated to 12 countries with a high malaria burden, and the rollout of these doses is expected to begin in early 2024.
While over 90% of malaria cases are confirmed in Africa, the Americas also confront various outbreaks.
On September 7, 2023, the Pan American Health Organization (PAHO) estimated that approximately 41 million people are living in areas where the risk of infection by malaria is considered moderate to high in 21 Latin American countries.
In the U.S., the Centers for Disease Control and Prevention issued a Health Alert Network Update (CDCHAN-00496) in August 2023 to share new information with clinicians, public health authorities, and the public about locally acquired malaria cases identified in Florida, Texas, and Maryland.
Gavi is a public-private partnership that helps vaccinate more than half the world's children against the world's deadliest diseases.
Our Trust Standards: Medical Advisory Committee