New Malaria Medication Option for International Travelers

For the first time in 18 years, the U.S. Food and Drug Administration (FDA) approved ARAKODA (tafenoquine) 100 mg tablets for oral use.
It is the first new medication in eighteen years for the prevention of malaria in patients 18+ years of age.
After an initial loading dose, ARAKODA is intended to be taken once a week, which could offer convenience to international travelers.
This announcement is important since 3.2 billion people live in 106 countries and territories at risk of malaria transmission.
The United States has approximately 1,700 cases of imported malaria occurring each year, with approximately 37 percent of these cases affecting women, including 5–6 percent who are pregnant at the time they are infected, says the Centers for Disease Prevention and Control (CDC).
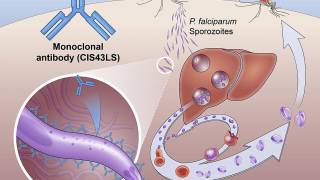
Tafenoquine is an 8-aminoquinoline chemically derived from Primaquine, with activity against all types of malaria. It was first synthesized by scientists at WRAIR in 1978.
Previously, on July 20th, the FDA approved single-dose Krintafel for the prevention of Plasmodium vivax (P vivax) malaria relapse in patients over the age of 16 years who are receiving antimalarial therapy.
Krintafel was the first drug to be approved for the treatment of P vivax in over 60 years.
ARAKODA’s approval was based on a concerted effort by the U.S. Army and 60 Degrees Pharmaceuticals (60P), involving over 21 clinical trials and over 3,100 trial subjects.
Since malaria is the top infectious disease threat to U.S. Military Service Members overseas, the military maintains a robust anti-malarial drug development effort through internal research and commercial partnerships.
Geoffrey Dow, Ph.D., the CEO of 60P, said: "ARAKODA provides the travel medicine community the option to prescribe an anti-malarial which provides protection in a large spectrum of malaria hot-zones while utilizing what is considered by many physicians to be a more compliant dosing regimen.”
“ARAKODA is a significant addition to the armamentarium for the prevention of malaria."
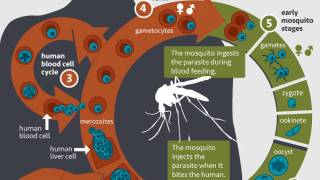
Malaria, a life-threatening disease transmitted through the bite of an infected mosquito, caused an estimated 429,000 fatalities and 212 million clinical cases in 2015, according to the Centers for Disease Control and Prevention (CDC).
60P has committed to the U.S. FDA to perform post-marketing safety surveillance studies to continue to gather data on this important tool against malaria.
ARAKODA™ should not be administered to:
- G6PD deficiency or unknown G6PD status
- Breastfeeding by a lactating woman when the infant is found to be G6PD deficient or if the G6PD status is unknown
- Patients with a history of psychotic disorders or current psychotic symptoms
- Known hypersensitivity reactions to tafenoquine, other 8-aminoquinolines, or any component of ARAKODA™
- G6PD testing must be performed before prescribing ARAKODA™ due to the risk of hemolytic anemia. Monitor patients for signs or symptoms of hemolysis.
- ARAKODA™ is not recommended during pregnancy.
- Serious psychotic adverse reactions have been observed in patients with a history of psychosis or schizophrenia, at doses different from the approved dose.
- Serious hypersensitivity reactions have been observed with administration of ARAKODA™. If hypersensitivity reactions occur, institute appropriate therapy.
- Due to the long half-life of ARAKODA™ (approximately 17 days), psychiatric effects, hemolytic anemia, methemoglobinemia, and hypersensitivity reactions may be delayed in onset and/or duration.
- The most common adverse reactions (incidence ≥1%) were: headache, dizziness, back pain, diarrhea, nausea, vomiting, increased alanine aminotransferase (ALT), motion sickness, insomnia, depression, abnormal dreams, and anxiety.
- Avoid co-administration with drugs that are substrates of organic cation transporter-2 (OCT2) or multidrug and toxin extrusion (MATE) transporters.
To report ARAKODA SUSPECTED ADVERSE REACTIONS, contact 60 Degrees Pharmaceuticals at 1-888-834-0225 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Separately, RTS,S/AS01 (RTS,S) is the world’s first malaria vaccine that has been shown to provide partial protection against malaria in young children in a phase 3 study.
This vaccine has been recommended by WHO for pilot introduction in selected areas of 3 African countries.
Our Trust Standards: Medical Advisory Committee