Yale Scientist Identifies Malaria Vaccine Target

A team of researchers has created a non-human vaccine that protects against malaria infection.
This is important since there is not a completely effective malaria vaccine available.
In 2013, there were approximately 200 million clinical cases and 584,000 deaths from malaria caused by parasites of the genus Plasmodium.
This Yale lead study paves the way for the development of a human vaccine that works by targeting the specific protein that parasites use to evade the immune system.
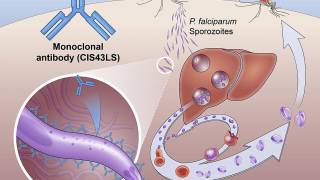
In a previous study Richard Bucala, M.D. described a unique protein produced by malaria parasites, Plasmodium macrophage migration inhibitory factor (PMIF), which suppresses memory T cells, the infection-fighting cells that respond to threats and protect the body against reinfection.
In this new study, Richard Bucala, M.D. and his co-authors collaborated with Novartis Vaccines, Inc. to test an RNA-based vaccine designed to target PMIF.
RNA delivery and stability improvements include RNA structure modifications, targeting dendritic cells and employing self-amplifying RNA.
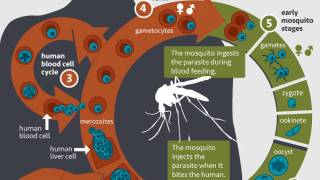
First, using a strain of the malaria parasite with PMIF genetically deleted, they observed that mice infected with that strain developed memory T cells and showed stronger anti-parasite immunity.
Next, the research team used two mouse models of malaria to test the effectiveness of a vaccine using PMIF.
One model had an early-stage liver infection from parasites carried by mosquitoes, and the other, a severe, late-stage blood infection.
In both models, the vaccine protected against reinfection.
As a final test, the researchers transferred memory T cells from the immunized mice to "naïve" mice never exposed to malaria.
Those mice were also protected.
The research shows, first, that PMIF is critical to the completion of the parasite life cycle because it ensures transmission to new hosts, said the scientists in a press release, noting it also demonstrates the effectiveness of the anti-PMIF vaccine.
"If you vaccinate with this specific protein used by the malaria parasite to evade an immune response, you can elicit protection against re-infection," said Dr. Bucala.
"To our knowledge, this has never been shown using a single antigen in fulminant blood-stage infection."
The next step for the research team is to develop a vaccine for individuals who have never had malaria, primarily young children.
"The vaccine would be used in children so that they would already have an immune response to this particular malaria product, and when they became infected with malaria, they would have a normal T cell response, clear the parasite, and be protected from future infection," Dr. Bucala stated.
The researchers also noted that because the PMIF protein has been conserved by evolution in different malaria strains and targets a host pathway, it would be virtually impossible for the parasite to develop resistance to this vaccine.
Numerous other parasitic pathogens also produce MIF-like proteins, said the scientists, suggesting that this approach may be generalizable to other parasitic diseases -- such as Leishmaniasis, Hookworm, and Filariais -- for which no vaccines exist.
The study was published by Nature Communications. Other authors are Alvaro Baeza Garcia, Edwin Siu, Tiffany Sun, Valerie Exler, Luis Brito, Armin Hekele, Gib Otten, Kevin Augustijn, Chris J. Janse, Jeff Ulmer, Jurgen Bernhagen, Erol Fikrig, and Andrew Geall.
This work was funded by National Institutes of Health grants and Novartis Vaccines, Inc.
Yale University and Novartis AG have filed a joint patent application describing the potential utility of a PMIF encoding RNA replicon. R.B. and A.G. are co-inventors on this application.
Our Trust Standards: Medical Advisory Committee