$9 Billion RSV Vaccine Market Share Battle Begins
After several years of development, various countries will offer infants, pregnant women, and seniors therapeutic options to prevent respiratory syncytial virus (RSV) infections in 2023.
Driven by recent governmental authorizations, sales of RSV drugs are estimated to surpass $9 billion by 2029, a sizeable increase from the $1 billion forcasted by GlobalData plc in 2023.
However, this forecast may change since each RSV season's duration is a variable. In the U.S., RSV seasons generally start in Florida.
According to this leading data and analytics company, May 2023 saw the world's first approval of two RSV vaccines, Arexvy™ and Abrysvo™.
The U.S. CDC now recommends that adults 60 and older may receive an RSV vaccine using shared clinical decision-making.
Additionally, AstraZeneca's Beyfortus™ monoclonal antibody (mAb) therapy was recently approved.
Jasper Morley, Drugs Intelligence Analyst at GlobalData, commented in a press release on July 24, 2023, "Until recently, there were no approved prophylactic treatments available."
"Approved by the U.S. FDA in 2023, GSK's Arexvy and Pfizer's Abrysvo are subunit vaccines indicated for preventing lower respiratory tract disease caused by RSV in individuals aged 60 and over."
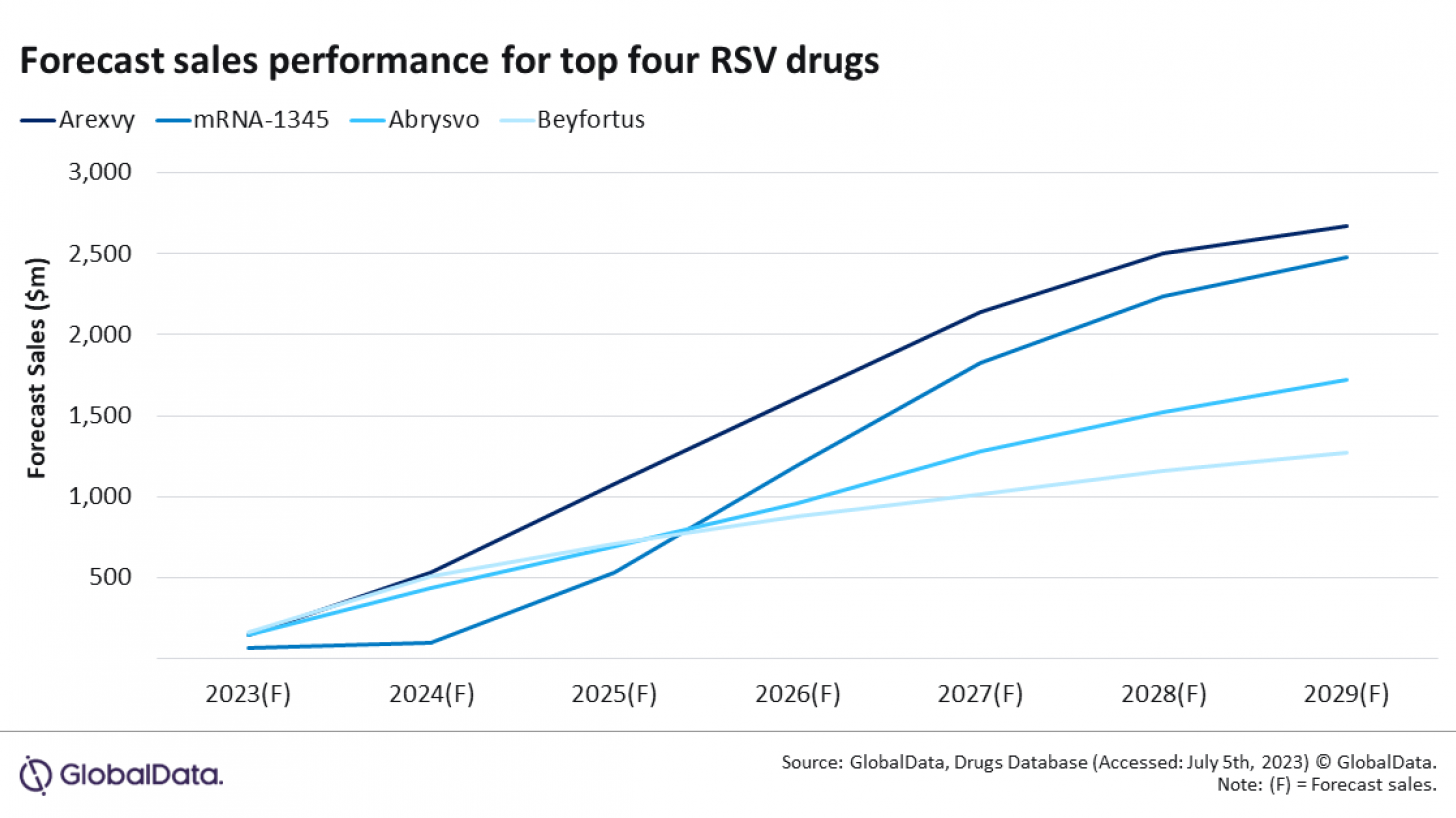
"Including Moderna's mRNA-1345 and AstraZeneca's Beyfortus, the four therapies will make up 90% of total forecast sales in 2029 and are the main drivers of the significant increase in the global RSV market."
As of July 24, 2023, other RSV vaccine candidates are conducting late-stage clinical trials.
Following U.S. and E.U. approval in May and June 2023, respectively, Arexvy is projected to climb steadily and emerge as a market leader, according to GlobaldData's Sales and Forecast tool.
This vaccine is forecast to retain the top spot over seven years, ultimately generating over a quarter of total global RSV market sales, with just over $2.5 billion in 2029.
Despite receiving U.S. approval in May, Abrysvo is still awaiting E.U. approval. It is forecast to achieve sales of $1.7 billion, securing third place behind Moderna's mRNA-1345 vaccine.
This mRNA vaccine candidate is expected to launch in 2024 and experience exponential growth to reach sales of $2.4 billion in 2029.
After receiving E.U. approval late last year and U.S. approval on July 17, 2023, Beyfortus is set to reach global sales of $1.27 billion. This mAb is the world's second treatment indicated for disease prevention in infants.
Morley concludes: "With the leading four RSV treatments all indicated prophylactically and eager to establish themselves before the fall season, the RSV market is poised to become a major pharmaceutical battleground over the next seven years."
"Fierce rivalry is to be expected, and the prospect of additional drug approvals, including geographical and maternal vaccinations, will grant treatments a competitive edge and a favorable position in the market."
According to the U.S. CDC, RSV is a common infectious disease of the lungs and respiratory tract that can cause further health problems such as bronchiolitis and pneumonia.
Although infections in healthy children and adults are less severe, certain patient groups, such as children with lung disease, the elderly, or the immunocompromised, may experience life-threatening symptoms.
Our Trust Standards: Medical Advisory Committee
























