Pregnant Women Prefer RSV Passive Immunization Protection for Infants
In 2023, the U.S. Food and Drug Administration (FDA) approved one respiratory syncytial virus (RSV) vaccine and an updated monoclonal antibody therapy to prevent respiratory disease in very young children.
Given these were new options, health officials did not know which product pregnant women would prefer during the 2023-2024 RSV season.
According to new data published by the U.S. Centers for Disease Control and Prevention (CDC) on January 23, 2024, the winner has been Beyfortus™ (Nirsevimab).
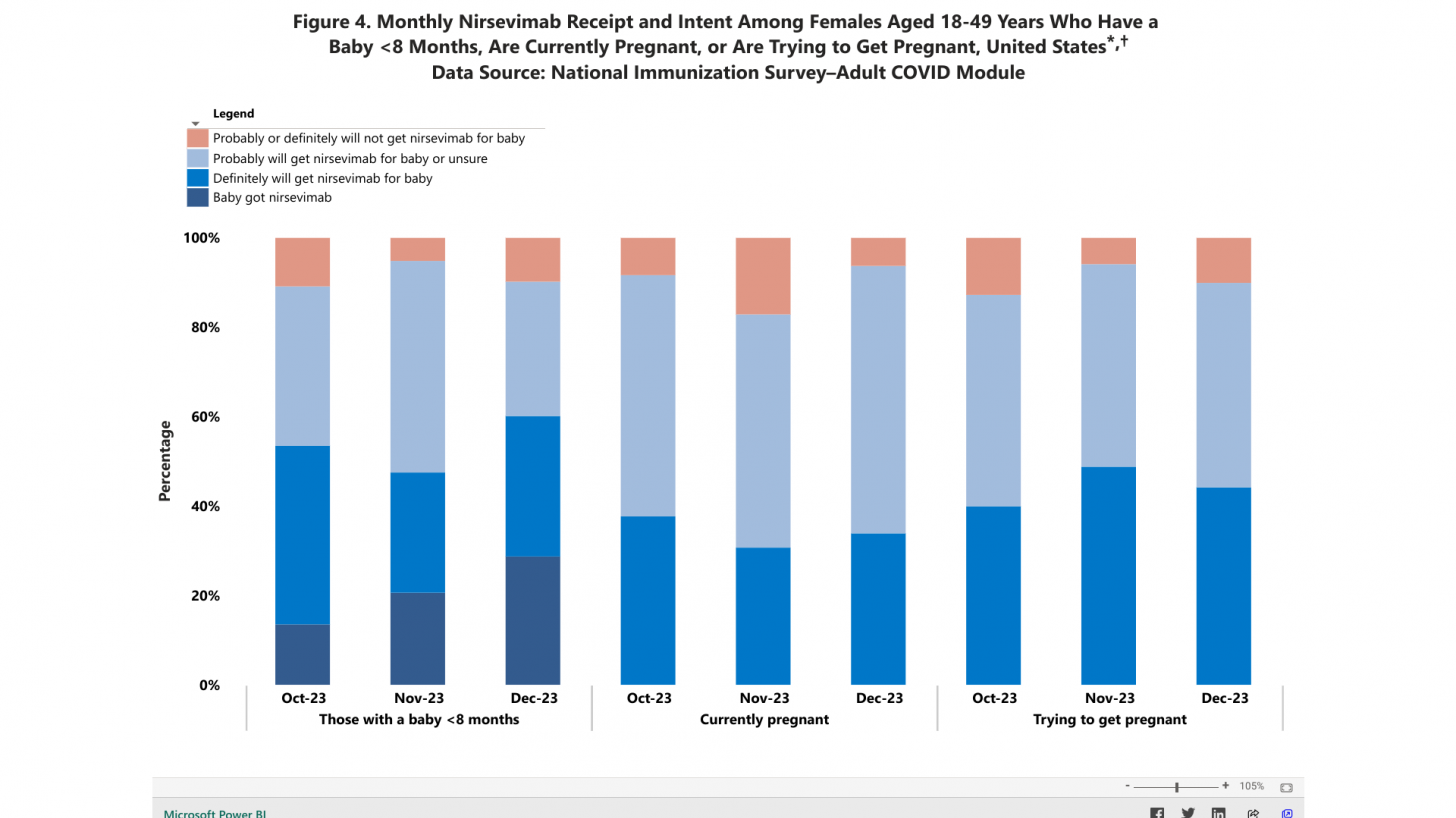
As of December 2023, among mothers with an infant younger than eight months, 28.7% reported that their infant received Beyfortus, and 33.9% reported that they plan to get Beyfortus for their infant.
Among women 18-49 years of age trying to get pregnant, 44.2% reported planning to get Beyfortus for their expected child.
Regarding vaccination, among women who were pregnant and ≥32 weeks gestation since September 22, 2023, the overall coverage with the RSV vaccine was 14.4% as of January 13, 2024.
Beyfortus is an extended half-life monoclonal antibody offering passive immunization to prevent lower respiratory tract infections.
Monoclonal antibodies do not activate the human immune system, as would occur with vaccination. Instead, the antibodies themselves protect against disease, which is called passive immunization.
The FDA recommends it for all infants younger than eight months of age who are born during—or entering—their first RSV season.
While the timing of each RSV season in the U.S. varies geographically, Beyfortus may be administered from October through the end of March in most of the continental, says the U.S. CDC.
Beyfortus is also recommended for some children aged 8 through 19 months who are at increased risk for severe RSV disease and entering their second RSV season.
Except in rare circumstances, the CDC says that most infants younger than eight months do not need Beyfortus if they were born 14 or more days after their mother got the RSV vaccine.
The U.S. FDA first approved Synagis® (Palivizumab), a multi-dose injectable RSV antibody that provides one month of protection, in 1998.
Our Trust Standards: Medical Advisory Committee
























