Third RSV Vaccine Seeks Various Approvals

As the 2023 Respiratory Syncytial Virus (RSV) season approaches the United States, a third vaccine may soon become available for older adults.
Moderna, Inc. today provided an update on regulatory submissions for mRNA-1345, a vaccine candidate for the prevention of RSV-associated lower respiratory tract disease (RSV-LRTD) and acute respiratory disease (ARD) in adults aged 60 years or older.
Moderna confirmed it has submitted marketing authorization applications for mRNA-1345 with the European Medicines Agency, Swissmedic in Switzerland, and the Therapeutic Goods Administration in Australia.
Additionally, the Company has initiated the rolling submission process for a Biologics License Application to the U.S. Food and Drug Administration (FDA) for the licensure of the mRNA-based RSV vaccine.
The FDA previously granted Fast Track and Breakthrough Designation designations for mRNA-1345.
Stéphane Bancel, Chief Executive Officer of Moderna, commented in a press release on July 5, 2023, "Our mRNA platform has allowed us to move from initial clinical testing to our first international Phase 3 trial to initiation of regulatory submissions for mRNA-1345 in just two years, enabling us to tackle this pervasive public health burden with speed and clinical rigor."
"mRNA-1345 represents the second product coming from our mRNA platform to seek global approval, and with recent positive data in rare disease and cancer, we expect more in the future - further demonstrating the tremendous potential of mRNA to combat disease."
The regulatory applications are based on positive data from a prespecified interim analysis of the pivotal ConquerRSV study, a randomized, double-blind, placebo-controlled study of approximately 37,000 adults 60 years or older in 22 countries.
In addition to older adults, mRNA-1345 is being investigated in a fully enrolled, ongoing Phase 1 trial in pediatric populations.
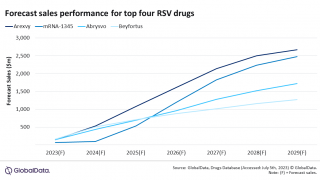
In the U.S., the Centers for Disease Control and Prevention (CDC) announced on June 29, 2023, its recommendation for the use of two previously approved RSV vaccines (AREXVY™, ABRYSVO™) for people ages 60 years and older, using shared clinical decision-making.
Most people with RSV infection will have mild illness and recover in a week or two.
However, some people, such as older adults, are more likely to develop severe RSV infection and may need to be hospitalized, says the CDC.
Recently, the World Health Organization Influenza Update N° 448 found RSV activity was found to be generally low globally except in Australia and a few countries in the Region of the Americas.
In the U.S., the RSV season generally starts in Florida.
As of June 24, 2023, RSV activity in Florida was low, but an increased positivity rate.
As of July 5, 2023, other RSV vaccine candidates are conducting late-stage clinical trials.
Our Trust Standards: Medical Advisory Committee