Single-Dose Malaria Relapse Pill Approved in Australia

Medicines for Malaria Venture (MMV) recently announced that the Australian Therapeutic Goods Administration (TGA) had approved the use of single-dose Kozenis (tafenoquine) in children in combination with chloroquine for the prevention of relapse of Plasmodium vivax (P. vivax) malaria.
The TGA approval confirmed on March 14, 2022, includes a novel, 50 mg dispersible tablet that can be dispersed in water and developed by GSK in partnership with MMV to facilitate use in children who are disproportionately affected by the disease.
P. vivax malaria is estimated to cause about 5 million clinical infections every year. It poses a disproportionate burden for children aged 2 to 6 years who are four times as likely as adults to be infected.
“We are proud to have worked with GSK to develop this child-friendly treatment and are thrilled by today’s announcement,” said Dr. David Reddy, Chief Executive Officer, MMV, in a press statement.
“P. vivax malaria is particularly dangerous for young children for whom repeated relapses can lead to cumulative severe anemia and, in some cases, be fatal.”
“Today, we have a tool to put a stop to the relentless relapse both for adults and children – we are one step closer to defeating this disease.”
TGA’s approval was supported by a Phase 2b clinical study that evaluated dosages of tafenoquine based on weight for children between the age of 2 years and weighing at least 10 kg, and up to 15 years.
The Lancet published the interpretation from this study in December 2021: For the prevention of P vivax relapse in children, single-dose tafenoquine, including a dispersible formulation, had exposure, safety, and efficacy consistent with observations in adolescents and adults, notwithstanding post-dose vomiting.
Kozenis is a single-dose treatment for preventing relapse of P. vivax and was approved for people aged 16 years and older by the TGA in 2018.
It should be used with a course of chloroquine to treat the active blood-stage infection, thereby achieving a radical cure.
The current standard of care for preventing P. vivax relapse requires a 7- or 14-day course of treatment with a drug called primaquine.
At present, there are no quality-assured, age-specific pediatric formulations marketed.
P. vivax malaria infections also impact a child’s development and educational progress. In addition, evidence shows that children who experience repeated P. vivax infections are likely to suffer from physical and cognitive impairment.
Further regulatory submissions are planned in malaria-endemic countries for pediatric indications for tafenoquine.
Furthermore, there is a preventive vaccine for malaria approved.
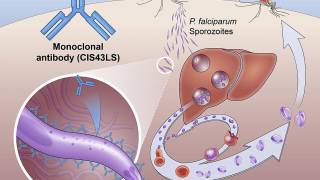
On October 6, 2021, the WHO recommended the RTS,S malaria vaccine for sub-Saharan Africa and other regions with moderate to high malaria transmission.
GSK’s Mosquirix RTS,S/AS01 vaccine aims to trigger the immune system to defend against the first stages when the Plasmodium falciparum malaria parasite enters the human host's bloodstream through a mosquito bite and infects liver cells.
Additional malaria vaccine development news is posted at Vax-Before-Travel/malaria.
Vax-Before-Travel publishes fact-checked, research-based travel vaccine and medicine news.
Note: This news article integrates content from various sources, edited for clarity and curated for mobile readers.
Our Trust Standards: Medical Advisory Committee
- Single-dose Kozenis approved for children with Plasmodium vivax malaria by Australian Therapeutic Goods Administration
- Tafenoquine exposure assessment, safety, and relapse prevention efficacy in children with Plasmodium vivax malaria
- A Pharmacokinetics, Safety and Efficacy Study of Tafenoquine (TQ) in Pediatric Subjects With Plasmodium Vivax (P. Vivax) Malaria
- Malaria Vaccines for 2022