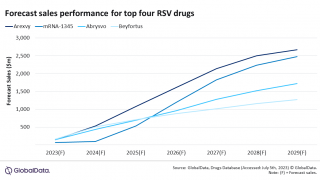
RSV Phase 3 Study Reaches Enrollment Milestone

A Phase 3 study for respiratory syncytial virus F protein recombinant nanoparticle vaccine (RSV F Vaccine) announced that it has reached approximately 4,600 participants, of whom at least 3,000 have received the RSV F Vaccine.
This is an important milestone because RSV remains an urgent global unmet medical need due to infant mortality and morbidity associated with RSV disease.
Moreover, there is not an approved vaccine to prevent RSV.
In the USA, RSV is the leading cause of hospitalization of infants.
Novavax, Inc.’s Prepare™ is a global, pivotal Phase 3 clinical trial of the RSV F Vaccine, in healthy, third-trimester pregnant women.
In December 2017, Novavax conducted an informational analysis related to the prevention of medically significant RSV-positive LRTI in a subset of 1,300 infants from the Prepare trial.
This analysis allows Novavax to conclude that the vaccine’s potential observed efficacy in this subset group is in the range of 45% and 100%.
The primary objective of the Prepare trial is to determine the efficacy of maternal immunization with the RSV F Vaccine against medically significant RSV-positive lower respiratory tract infection (LRTI) in infants through a minimum of the first 90 days of life, and up through the first 6 months of life.
Stanley C. Erck, President, and CEO of Novavax. Inc. said Novavax will initiate a prespecified interim efficacy analysis for the Prepare trial after the last infant born to the approximately 4,600 women enrolled in the trial has been followed for six months.
Novavax expects to report on the interim data in the first quarter of 2019.
Assuming successful interim analysis results, the trial would be concluded without further enrollment and Novavax would file a biologics license application (BLA) with the U.S. Food and Drug Administration (FDA) and a marketing authorization application (MAA) with the European Medicines Agency (EMA) by the first quarter of 2020.
The Prepare trial is supported by a grant of up to $89.1 million from the Bill & Melinda Gates Fund. This grant supports development activities, product licensing efforts and World Health Organization prequalification of the RSV F Vaccine.
RSV is the most common cause of lower respiratory tract infections and the leading viral cause of severe lower respiratory tract disease in infants and young children worldwide, with estimated annual infection and mortality rates of 64 million and 160,000, respectively.
For more information, please visit www.novavax.com.
Our Trust Standards: Medical Advisory Committee