55,000 Sudan Ebolavirus Vaccine Candidates Coming by Year End

The nonprofit scientific research organization IAVI and Merck & Co., Inc. announced today they have entered into an agreement that could enable IAVI to accelerate the entry of a Sudan ebolavirus (SUDV) vaccine candidate that could be deployed in the Republic of Uganda this year.
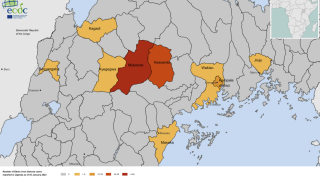
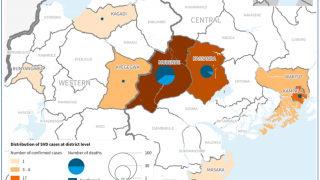
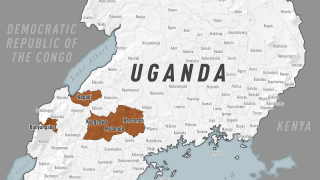
The Ministry of Health of Uganda has declared a SUDV outbreak in several districts that have claimed about 28 lives since late September 2022.
Merck confirmed on October 25, 2022, plans to produce and provide vials of candidate vaccines from existing investigational drug substance to IAVI to supplement IAVI's ongoing SUDV vaccine development program.
Science.org reported on October 23, 2022, that Merck had about 100,000 potential doses in a Pennsylvania facility.
The new investigational vaccine is based on the same vesicular stomatitis virus viral vector platform in ERVEBO®, Merck's single-dose Zaire ebolavirus vaccine that has been approved by the U.S. FDA and several other regulatory authorities.
Mark Feinberg, M.D., Ph.D., president and CEO of IAVI, commented in a related press release, "We are grateful to Merck for supplying the vaccine material, and we look forward to the opportunity to demonstrate vaccine effectiveness and safety so that we are prepared for future outbreaks of SUDV, as well as the SUDV outbreak in Uganda should it not be promptly contained by public health measures alone."
However, the actual vaccine production schedule is still being defined.
Based on the quantities of available bulk drug substance and current plans, Merck stated it hopes to be able to deliver approximately 55,000 doses by the end of 2022.
The number of doses provided by Merck should be sufficient for conducting Phase I and efficacy studies as well as for public health response if the outbreak in Uganda continues or spreads and should the vaccine candidate be found efficacious.
IAVI will act as developer and regulatory sponsor and will be responsible for all aspects of the future development of the vaccine candidate.
IAVI is additionally working to accelerate the manufacture of doses of IAVI's VSV-SUDV vaccine should they be needed.
Other SUDV vaccine candidates have been announced, which are listed at PrecicionVaccinations.com/Ebola.
PrecisionVaccinations publishes fact-checked, research-based vaccine news manually curated for mobile readers.
Our Trust Standards: Medical Advisory Committee