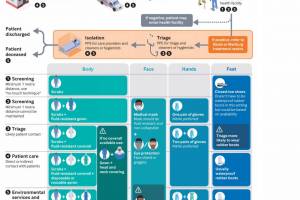
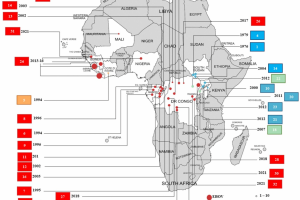
Ebola Vaccines
Ebola Vaccines 2024
Since 2014, Ebola vaccine technologies have included replication-deficient adenovirus vectors, replication-competent vesicular stomatitis, human parainfluenza vectors, and virus-like nanoparticle preparations. The World Health Organization (WHO) has approved Ebola vaccines to protect people against Ebolavirus outbreaks since 2019. The U.S. Biomedical Advanced Research and Development Authority (BARDA) has funded and is developing vaccine candidates against the six filoviruses. In 2021, the International Coordinating Group on Vaccine Provision established a global Ebola vaccine stockpile.
Zaire Ebolavirus Vaccines 2024
Various Zaire Ebolavirus vaccines have been approved by the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), the WHO, and the United Kingdom. As of July 2024, the U.S. Centers for Disease Control and Prevention (CDC) says two licensed vaccines are recommended for the prevention of Ebola caused by Orthoebolavirus zairense: the rVSVΔG-ZEBOV-GP (ERVEBO) and the Ad26.ZEBOV and MVA-BN-Filo.
Merck's Ervebo® vaccine
Johnson & Johnson Zabdeno and Mvabea (Ad26.ZEBOV, MVA-BN-Filo) ebola vaccine regimem
CanSinoBio's Ad5-EBOV vaccine
U.S. NIAID/NIH with Okairos produced cAd3-EBOZ/MVA-BN-Filo vaccine
Zaire Ebolavirus Vaccine Candidates
INOVIO's INO-4201 is a DNA vaccine candidate targeting Zaire Ebola virus glycoprotein, designed to prevent ZEBOV infection. INO-4201 was evaluated in a Phase 1b clinical trial. On May 11, 2023, the Company announced that INO-4201 was well-tolerated and boosted humoral responses in 36 36 (100%) treated participants as an Ebola booster for ERVEBO®.
S.K. bioscience entered into a development licensing agreement with Hilleman Laboratories in November 2023 for joint development of a second-generation Zaire Ebola virus vaccine. "We look forward to working closely with MSD to accelerate broad access to the second-generation Zaire ebolavirus vaccine and help to improve vaccine coverage for populations in LMICs," said Dr. Raman Rao, CEO of Hilleman Laboratories.
biEBOV bivalent adenovirus vectored vaccine is conducting a phase 1 study (NCT05301504). The Sabin Vaccine Institute USA produces the ChAd3-SUDV monovalent adenovirus vector vaccine.
YF-EBO is a live YF17D-vectored dual-target vaccine candidate expressing EBOV glycoprotein (G.P.) as a protective antigen. A single dose of YF-EBO was sufficient to induce high levels of EBOV GP-specific antibodies and cellular immune responses that protected against lethal infection using EBOV GP-pseudotyped recombinant vesicular stomatitis virus (rVSV-EBOV) in interferon-deficient (Ifnar-/-) mice as surrogate challenge model. Concomitantly induced yellow fever virus (YFV)-specific immunity protected Ifnar-/- mice against intracranial YFV challenge.
INOVIO announced on April 12, 2023, that an abstract had been accepted for presentation for INO-4201 as an Ebola booster for rVSV-ZEBOV (Ervebo) at the 33rd European Congress of Clinical Microbiology and Infectious Diseases.
Sudan Ebolavirus Vaccine Candidates
As of July 2024, no approved vaccines protect people against the Sudan Ebolavirus (SUDV). However, the WHO confirmed candidate vaccines are being tested in the Solidarity Against Ebola human clinical studies.
The Sabin Vaccine Institute is developing a single-dose vaccine candidate for Sudan Ebolavirus. Based on the same cAd3 platform as its Marburg vaccine candidate, Sabin’s Sudan ebolavirus vaccine was found to be promising in Phase 1 clinical and non-clinical studies, with results showing it to be safe while eliciting rapid and robust immune responses that lasted up to 12 months.
IAVI's rVSV Sudan ebolavirus vaccine candidate (IAVI C108, rVSVΔG-SUDV-GP) was confirmed on June 27, 2023. The first participants were vaccinated with a SUDV vaccine candidate in a first-in-human Phase I clinical trial in the U.S. Previously, BARDA issued financial awards to IAVI to support IAVI's SUDV vaccine candidate.
Serum Institute of India experimental Sudan Ebolavirus vaccine.
GeoVax Labs, Inc.'s vectored vaccine MVA-VLP-SUDV was generated against Sudan Ebolavirus. Data published on July 25, 2022, demonstrate the MVA-VLP platform's single-dose protection and potency for use in emergencies to contain outbreaks.
Soligenix, Inc. proposes developing SuVax™, a single-vial, adjuvanted, heat-stable subunit vaccine to prevent filovirus infection for use in the event of a Sudan ebolavirus outbreak. On September 25, 2023, the thermostabilized bivalent and trivalent filovirus vaccine candidates demonstrated two-year stability at temperatures of 40 degrees Celsius when formulated in a single vial, needing reconstitution only with sterile water immediately before use. The filovirus vaccines represent the only recombinant subunit vaccines to date that have demonstrated complete protection against challenges with Zaire ebolavirus, Sudan ebolavirus, and Marburg marburgvirus in NHPs.
The U.S. government initially invested $35 million to produce up to 100,000 doses of ChAd3-SUDV. These vaccines may be part of ongoing U.S. preparedness efforts and response to future global outbreaks.
The National Institutes of Health Rocky Mountain Laboratories in Hamilton, Montana, developed a candidate for vesicular stomatitis virus-based Sudan virus vaccine (VSV-SUDte. The investigators anticipate that giving people VSV-SUDV in a dosage similar to that of VSV-EBOV would provide rapid protective immunity to SUDV.
VRC-EBOADC086-00-VP, a chimpanzee adenovirus serotype 3 vector-based Ebola vaccine, encodes wild-type glycoprotein from the Sudan strain of Ebolavirus and is administered intramuscularly. A Phase I Open-Label, Dose-Escalation Clinical Trial to Evaluate Two Doses Safety, Tolerability, and Immunogenicity. In August 2023, a study found the cAd3-EBO S vaccine was safe at both doses, rapidly inducing immune responses in most participants after a single injection. The rapid onset and durability of the vaccine-induced antibodies make this vaccine a strong candidate for emergency deployment in SDV outbreaks.
Ebolavirus Passive Immuninzation
The Lancet Infectious Disease reported on November 30, 2023, that monoclonal antibodies (mAbs) were approved for emergency use by the U.S. FDA and used in clinical trials during the DRC 2018–20 EVD epidemic. These trials demonstrated that mAbs contributed to an increase in the number of EVD survivors. The WHO recommended that in 2022, two monoclonal antibody treatments be used in treating Ebola: mAb114 (Ansuvimab; Ebanga) and REGN-EB3 (Inmazeb). The U.S. National Institutes of Health (NIH) says monoclonal antibodies (mAbs) are made in a laboratory to fight a particular infection and are administered during an infusion. mAbs are different than vaccines, says the NIH. Approved Ebola mAbs included but are not limited to:
Ebanga (mAb114) is a human IgG1 MAb targeted to the Zaire ebolavirus glycoprotein, available in a lyophilized form. The WHO issued a strong recommendation in August 2022. On December 22, 2020, Ridgeback Biotherapeutics L.P. confirmed that the U.S. FDA approved Ebanga to treat Zaire Ebola.
On May 2, 2022, the U.S. FDA issued a priority review voucher for a material threat medical countermeasure (MCM) product application for INMAZEB (atoltivimab, maftivimab, and odesivimab-ebgn), manufactured by Regeneron Pharmaceuticals, Inc. As a result, the FDA approved Inmazed in October 2020.
On October 14, 2020, the U.S. FDA approved an antibody cocktail from Regeneron that's been shown to reduce Ebola-related mortality rates. The treatment, known as REGN-EB3, is a mixture of (3) monoclonal antibodies (atoltivimab, maftivimab, and odesivimab-ebgn) and is marketed under the brand name Inmazeb. Inmazeb is indicated to treat infection caused by Zaire ebolavirus in adult and pediatric patients, including neonates born to a mother who is RT-PCR positive for Zaire ebolavirus infection.
The U.S. Administration for Strategic Preparedness and Response announced on October 4, 2022, a $109.8 million contract with Mapp Biopharmaceutical Inc. for the advanced development and potential purchase of a mAb therapy to treat Sudan Ebolavirus.
The Lancet Infectious Disease published results from an observational cohort study on November 30, 2023, that concluded almost 25% of survivors were seronegative on discharge from the Ebola treatment center, and antibody concentrations decreased rapidly over time. These results indicate that monoclonal antibodies might negatively affect the production of anti-Ebola virus antibodies in survivors of Ebola virus disease, which could increase the risk of reinfection or reactivation.
Ebolavirus Treatments
On April 8, 2024, RedHill Biopharma announced that its two novel, oral host-directed investigational drugs, opaganib and RHB-107 (upamostat), demonstrated robust synergistic effect when combined individually with remdesivir (Veklury®), significantly improving viral inhibition while maintaining cell viability. Opaganib (ABC294640) is a first-in-class, orally administered sphingosine kinase-2 selective inhibitor with the potential for broad activity across radioprotection, cancer, inflammatory, and viral conditions. Opaganib's development is focused on a potential role as a nuclear and chemical medical countermeasure in the event of radiation and chemical incidents.
Opaganib is believed to be the first host-directed molecule to show activity in Ebola virus disease, having recently delivered a statistically significant increase in survival time in a separate U.S. Army-funded in vivo Ebola virus study. When administered to the animals within 24 hours of virus exposure once daily for ten days, the drug conferred complete protection against lethal infection with Sudan ebolavirus.
RHB-107 was recently accepted for inclusion in the ACESO PROTECT adaptive platform trial for early COVID-19 outpatient treatment. The 300-patient PROTECT Phase 2 RHB-107 arm has received FDA clearance to start. The study is being conducted in the U.S., Thailand, Ivory Coast, South Africa, and Uganda and is estimated to be completed by the end of 2024. RHB-107 successfully met its U.S. Phase 2 study primary endpoint of safety and tolerability and delivered promising efficacy results, including a marked reduction in hospitalization due to COVID-19.