Uganda Unlocks Following Recent Sudan Ebola Outbreak
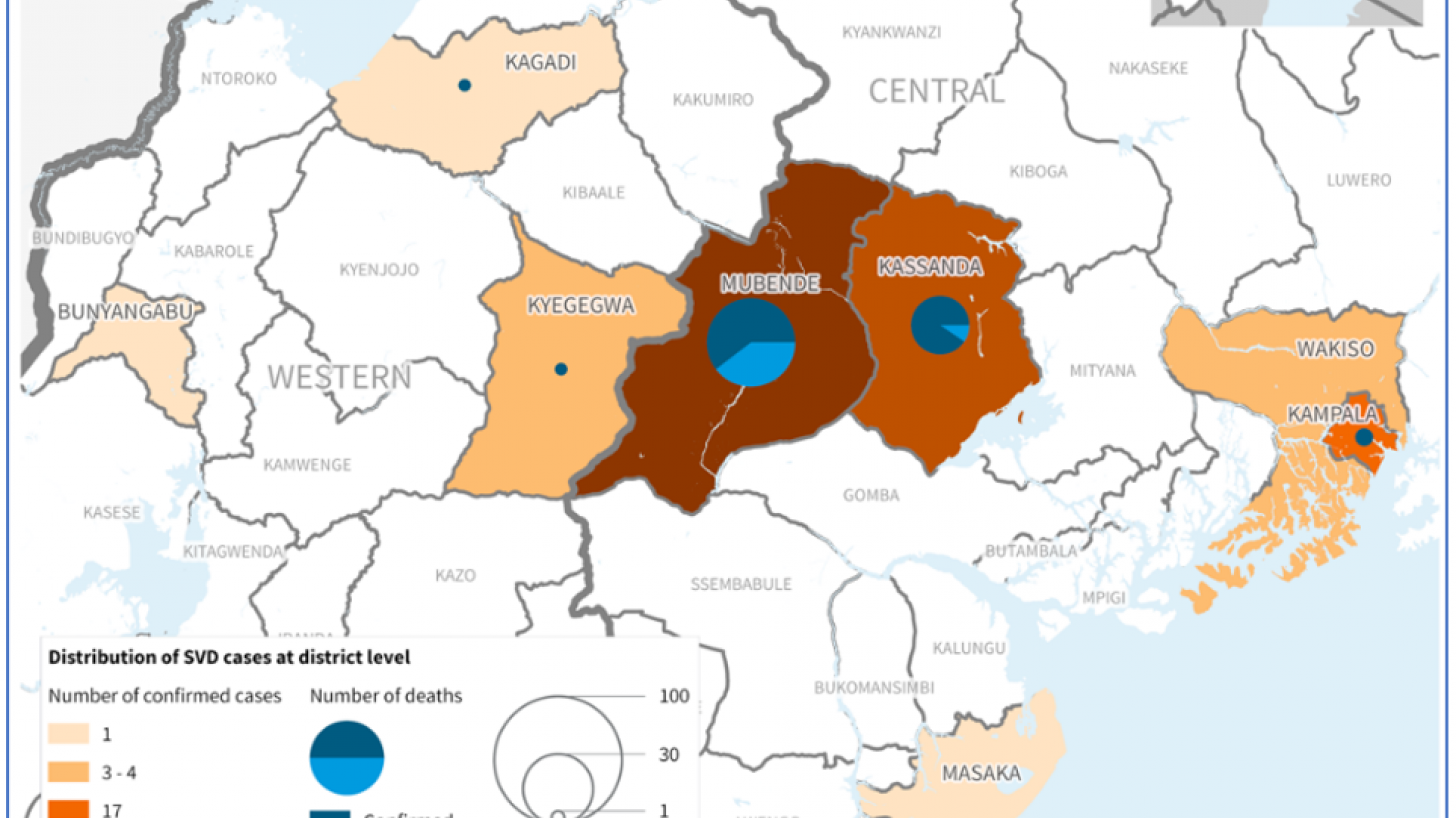
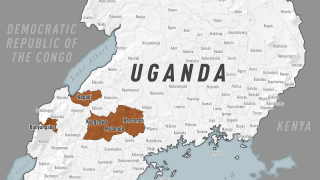
The Voice of America (VOA) reported yesterday the Republic of Uganda's Vice President Jessica Alupo announced that the government was immediately lifting all movement restrictions and curfews in the Mubende and Kassanda districts regarding the Sudan Ebolavirus (SUDV) outbreak.
Ugandan authorities announced last month that new SUDV cases were decreasing, and the last confirmed patient was discharged at the end of November.
The current SUDV outbreak was declared on September 20, 2022.
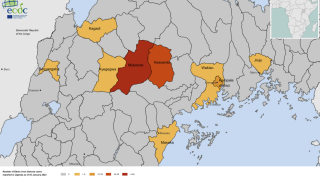
Since then, Uganda has reported 142 confirmed SUDV cases and 56 deaths, spreading the disease to the capital city of Kampala, which has over 1.5 million residents, says the World Health Organization (WHO).
To reduce the potential for spreading the SUDV, Kampala's airport has been screening outbound travelers.
And in the U.S., arriving air passengers from Uganda have also been screened for the virus.
On November 1, 2022, the WHO revised its risk assessment for this SUDV outbreak from high to very high at the national level and from low to high at the regional level, while the risk remained low at the global level.
The last SUDV outbreak in Uganda was reported in 2012.
"The lifting of the restrictions is based on the fact that no transmission, no contact under follow-up, no patients are in the isolation facilities," Alupo commented in a televised address delivered on December 17, 2022, reported VOA.
However, Alupa warned that the government remained on "high alert" for any resurgence in cases.
The good news announced recently by the WHO is there are human clinical trials underway evaluating SUDV vaccine candidates.
The WHO has scheduled a briefing for these studies on January 12, 2023.
While U.S. Food and Drug Administration (FDA) vaccines target the Zaire Ebolavirus, one is not approved for the SUDV.
The Ervebo® Ebola Vaccine was FDA-approved in 2019 and Listed by the WHO.
It is a live, recombinant, replication-competent vaccine and was genetically engineered to express the main glycoprotein from the Zaire ebolavirus to provoke a neutralizing immune response to Ebolavirus infections.
Additional Ebola outbreak and vaccine development news are posted at Vax-Before-Travel.com/Ebola.
Vax-Before-Travel publishes fact-checked, research-based travel vaccine information manually curated for mobile readers.
Our Trust Standards: Medical Advisory Committee