U.S. Prepares for Sudan Ebolavirus Potential Arrival

The U.S. CDC's Clinician Outreach and Communication Activity call on October 12, 2022, offered an emergency update on Uganda's ongoing Sudan Ebolavirus (SUDV) outbreak.
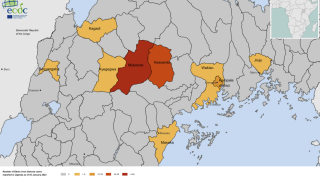
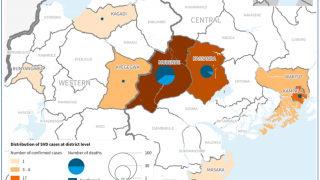
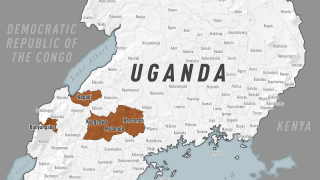
Led by Trevor Shoemaker, Ph.D., MPH, and Mary Choi, MD, MPH, this CDC presentation call reviewed the SUDV outbreak in Mubende District, which was declared on September 20, 2022.
Since then, Uganda's Ministry of Health has confirmed nineteen people have died from SUDV, indicating a case fatality rate of about 40%.
Before 2022, there were seven SUDV outbreaks, with 792 cases and 426 related deaths.
While Uganda focuses on its current SUDV outbreak, the World Health Organization has elevated this situation as a potential risk to other countries.
This health risk has been accessed as low by the WHO and the CDC due to source control measures at local airports and in the U.S.
About 140 travelers from Uganda arrive in the U.S. daily.
These passengers are now funneled into five U.S. airports for SUDV screening.
These travelers are followed-up post arrival by the U.S. government.
However, since SUDV infection incubation periods can reach seven days, airport screening may miss infected passengers, said the CDC team.
In the U.S., the CDC has mobilized various resources, such as multi-disciplinary CDC Ebola Response Teams and coordinating with the 10 Regional Special Pathogens Treatment Centers.
Currently, nine laboratories can test SUDV samples.
Furthermore, the CDC is activating 28 Laboratory Response Network laboratories to extend local preparations.
Unfortunately, there are no U.S. FDA-approved SUDV vaccines or treatments as of October 13, 2022.
However, the MBP134 experimental antibody cocktail therapy has demonstrated efficacy in preventing mortality due to infection with SUDV in non-human primates.
And there are two experimental vaccine candidates (University of Oxford; Sabin) preparing to undergo evaluation in Uganda.
But, available evidence suggests Ervebo® (rVSVΔG-ZEBOV-GP), the FDA-licensed vaccine against the Zaire Ebolavirus strain, would not provide cross-protection against a SUDV infection.
During this CDC call, an important suggestion for U.S.-based healthcare providers, when presented with a potential SUDV patient, collect travel history for ill patients presenting with a clinical picture suggestive of an infectious etiology.
To notify healthcare providers and international travelers of their potential health risks, the CDC issued an Alert - Level 2, Practice Enhanced Precautions, regarding Uganda's outbreak on October 4, 2022.
The CDC says travelers should isolate immediately and seek medical care if they develop signs and symptoms like fever, muscle pain, sore throat, diarrhea, weakness, vomiting, stomach pain, or unexplained bleeding or bruising during or for up to 21 days after travel.
The CDC staff indicated a severely SUDV-infected patient exhibits extensive vomiting.
As a resource for public health departments, the CDC's Viral Special Pathogens Branch is available 24/7 for consultations by calling CDC Emergency Operations Center (770-488-7100).
The full CDC call and presentation are linked.
PrecisionVaccinations publishes fact-based, research-based vaccine news manually curated for mobile readers.
Our Trust Standards: Medical Advisory Committee