$30 Million Funding Advances Marburg and Sudan Virus Vaccines

IAVI announced today that the U.S. Biomedical Advanced Research and Development Authority (BARDA) funded additional development and testing of single-dose vaccine candidates against the filoviruses Marburg virus (MARV) and Sudan ebolavirus (SUDV).
BARDA's US$30 million award to the nonprofit IAVI on March 2, 2023, expands the agency's existing support of SUDV vaccine candidate (rVSVΔG-SUDV-GP) development.
The new BARDA funding empowers IAVI and partners to advance the rVSVΔG-SUDV-GP development plan to include a Phase I clinical trial in the United States in 2023.
This funding is in addition to the initial award of $17 million in 2022 to advance IAVI's MARV vaccine candidate (rVSVΔG-MARV-GP) into human clinical trials.
These plans are enhanced following recent preclinical data demonstrating that a single dose of the vaccine was 100% efficacious at preventing Marburg virus disease (MVD) in nonhuman primates.
The Defense Threat Reduction Agency of the U.S. Department of Defense funded the nonclinical development of rVSVΔG-MARV-GP.
"Ongoing intermittent viral hemorrhagic fever outbreaks, including the recent SUDV and current MARV outbreaks, demonstrate the importance of developing and stockpiling vaccines against known threats so they are ready for rapid evaluation in clinical trials," said Swati Gupta, vice president and head of emerging infectious diseases and epidemiology at IAVI, in a press release on March 2, 2023.
"The funding from BARDA will enable us to continue critical development and testing of IAVI's filovirus vaccine candidates to facilitate better preparedness."
"It is essential that we efficiently accelerate our vaccine development efforts for known infectious disease threats now and apply our lessons learned from the past, such that we are truly better prepared when outbreaks continue to occur in the future."
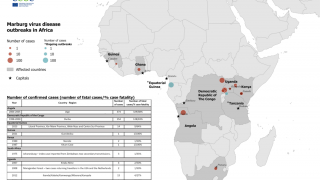
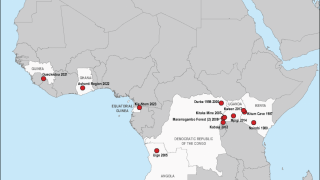
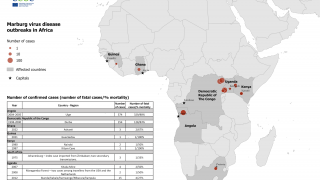
Previously, Filoviruses have caused severe hemorrhagic fever outbreaks in Central and Western Africa during 2022 and 2023.
IAVI's filovirus vaccine candidates are based on the same recombinant vesicular stomatitis virus (rVSV) vector used in ERVEBO®, a highly efficacious Ebola Zaire (ZEBOV) vaccine previously deployed by several African countries during Ebola virus outbreaks.
MARV is associated with sporadic African outbreaks and has a case fatality rate of up to 88%.
For example, Equatorial Guinea confirmed its first MARV outbreak in 2023, while Ghana and Guinea confirmed outbreaks in 2022 and 2021, respectively.
And in response to Uganda's SUDV outbreak in late 2022, the World Health Organization (WHO) vaccine prioritization working group assembled to rapidly evaluate the suitability of three candidate Ebola vaccines for inclusion in a planned ring vaccination trial in Uganda.
The WHO group ranked IAVI's rVSVΔG-SUDV-GP candidate the highest based on the proven safety and efficacy of ERVEBO®.
Vaccines against SUDV and MARV remain critical unmet needs because immunity against ZEBOV, SUDV, and MARV is not cross-protective.
This project is funded in whole or in part with federal funds from the Department of Health and Human Services, Administration for Strategic Preparedness and Response, Biomedical Advanced Research and Development Authority, under contract number 75A50121C00077.
IAVI is a nonprofit scientific research organization that addresses urgent, unmet global health challenges, including HIV, tuberculosis, and emerging infectious diseases. Read more at iavi.org.
Our Trust Standards: Medical Advisory Committee