Sabin Vaccine Institute Awarded $20.5 Million Dollars to Advance Ebola Sudan and Marburg Vaccines

The U.S. Department of Health and Human Services (HHS) announced it will support the simultaneous development of individual vaccine candidates against the Marburg virus and Sudan ebolavirus infections.
HHS said in a press release published on October 1, 2019, this support will enable the United States to prepare for and respond to these potential health security threats.
These viruses continue to pose potential public health and biodefense threats, says the HHS.
Specifically, the Biomedical Advanced Research and Development Authority (BARDA), part of the HHS, initially will provide The Albert B. Sabin Vaccine Institute Inc. with approximately $20.5 million dollars and technical expertise to develop these vaccine candidates.
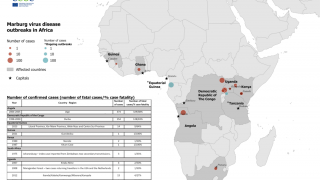
Both diseases are caused by potentially deadly filoviruses, the same family of viruses that includes the Ebola Zaire virus currently affecting communities in central Africa, such as the Democratic Republic of the Congo (DRC) and Uganda.
People can get these diseases through direct contact with an infected animal or a sick or dead person infected with either virus.
“The Sabin Vaccine Institute’s use of a single well-established platform for the Sudan ebolavirus and Marburg vaccines will help to expedite the clinical development process so that the vaccines are available more rapidly,” said BARDA Director Rick Bright, Ph.D., in the press release.
“In addition, by supporting the development of both vaccines simultaneously, BARDA is taking critical steps forward in protecting Americans from these potentially deadly viruses that could emerge and cause outbreaks at any time.”
Under the contract, BARDA has the option to provide additional funding to advance both vaccine candidates through Phase 2 clinical development.
Developing the vaccines simultaneously allows Sabin to use the same manufacturing platform, which could speed development and be more cost-effective than developing one vaccine and then the other independently.
The Sudan ebolavirus vaccine candidate is the first for this virus strain to receive BARDA support, and the Marburg vaccine candidate is the second to receive BARDA support.
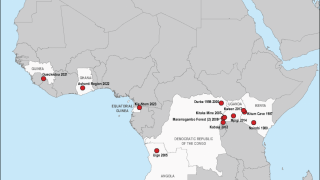
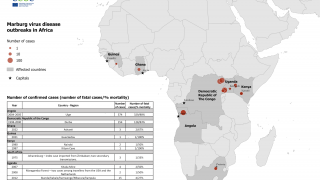
The most recent outbreaks of Marburg occurred in 2012, 2014 and 2017 in Uganda, Africa, where more than 25 percent of people infected died.
The last Sudan ebolavirus outbreak also took place in Uganda in 2012, killing 57 percent of people infected.
In addition to these two development projects, BARDA is supporting the development and procurement of vaccines, therapeutics, and diagnostics against the Ebola virus strain currently circulating in the DRC.
To date, Ebola has claimed more than 2,126 lives in DRC, with additional fatalities continuing to be reported.
Recent Ebola vaccine news
- Unknown Ebola Risk Discussed in Tanzania
- Ebola Treatment Receives $14 Million Dollar Advanced Development Support
HHS works to enhance and protect the health and well-being of all Americans, providing for effective health and human services and fostering advances in medicine, public health, and social services.
The mission of the Office of the Assistant Secretary for Preparedness and Response (ASPR) is to save lives and protect Americans from 21st-century health security threats.
Within ASPR, BARDA invests in the advanced research and development, acquisition, and manufacturing of medical countermeasures – vaccines, drugs, therapeutics, devices, diagnostic tools, and non-pharmaceutical products needed to combat health security threats.
For more about ASPR and BARDA, visit Public Health Emergency, and to learn more about partnering with BARDA.
Vaccine News Published by Precision Vaccinations
Our Trust Standards: Medical Advisory Committee