$214 Million Allocated for Ebola Sudan and Marburg Vaccines
The Sabin Vaccine Institute today announced that the U.S. Biomedical Advanced Research and Development Authority (BARDA) had awarded it a multi-year contract with funding potential for up to $214 million to advance the development and production of Ebola Sudan and Marburg virus diseases vaccines.
As of January 12, 2023, no licensed vaccines against Ebola Sudan and Marburg viruses exist.
However, if approved, these vaccines could be used as part of ongoing U.S. preparedness efforts and in response to future global outbreaks.
The new contract leverages a partnership with BARDA that began in 2019 when the agency awarded Sabin another multi-year contract valued at $128 million to further the development of Marburg and Ebola Sudan vaccines.
BARDA will initially invest approximately $35 million to produce up to 100,000 doses of Sabin’s Ebola Sudan virus (ChAd3-SUDV) vaccine.
The ChAd3-SUDV vaccine was the first to arrive in the Republic of Uganda during the recent Ebola Sudan virus outbreak, which ended on January 11, 2023.
The recent Sudan Ebolavirus (SUDV) outbreak in Uganda, the fifth overall, was declared on September 20, 2022. Since the outbreak declaration, there were 55 confirmed deaths, a case-fatality ratio of 47%.
The World Health Organization included ChAd3-SUDV as one of three vaccine candidates being tested in human clinical trials in Uganda.
And Sabin has initiated Phase 2 clinical trial planning in Uganda and Kenya.
Based on previous non-human clinical trials, Sabin’s Ebola Sudan vaccine was found safe and immunogenic.
“Sabin successfully delivered Ebola Sudan vaccine doses to Uganda within 79 days of the start of the outbreak – quite an impressive accomplishment,” says Sabin Chief Executive Officer Amy Finan in a related press release.
“This new contract enables Sabin to produce up to 100,000 doses, so the world is prepared for future outbreaks.”
Separate from the Sabin initiative, other SUDV vaccine candidates continue to be developed.
In addition, the BARDA contract also includes support to manufacture Sabin’s vaccine against the Marburg virus (ChAd3-MARV), which could be used in clinical trials and response to a possible Marburg virus outbreak.
As recently as last July 2022, two people in Ghana died after being infected with the Marburg virus, reinforcing the urgent need for a vaccine.
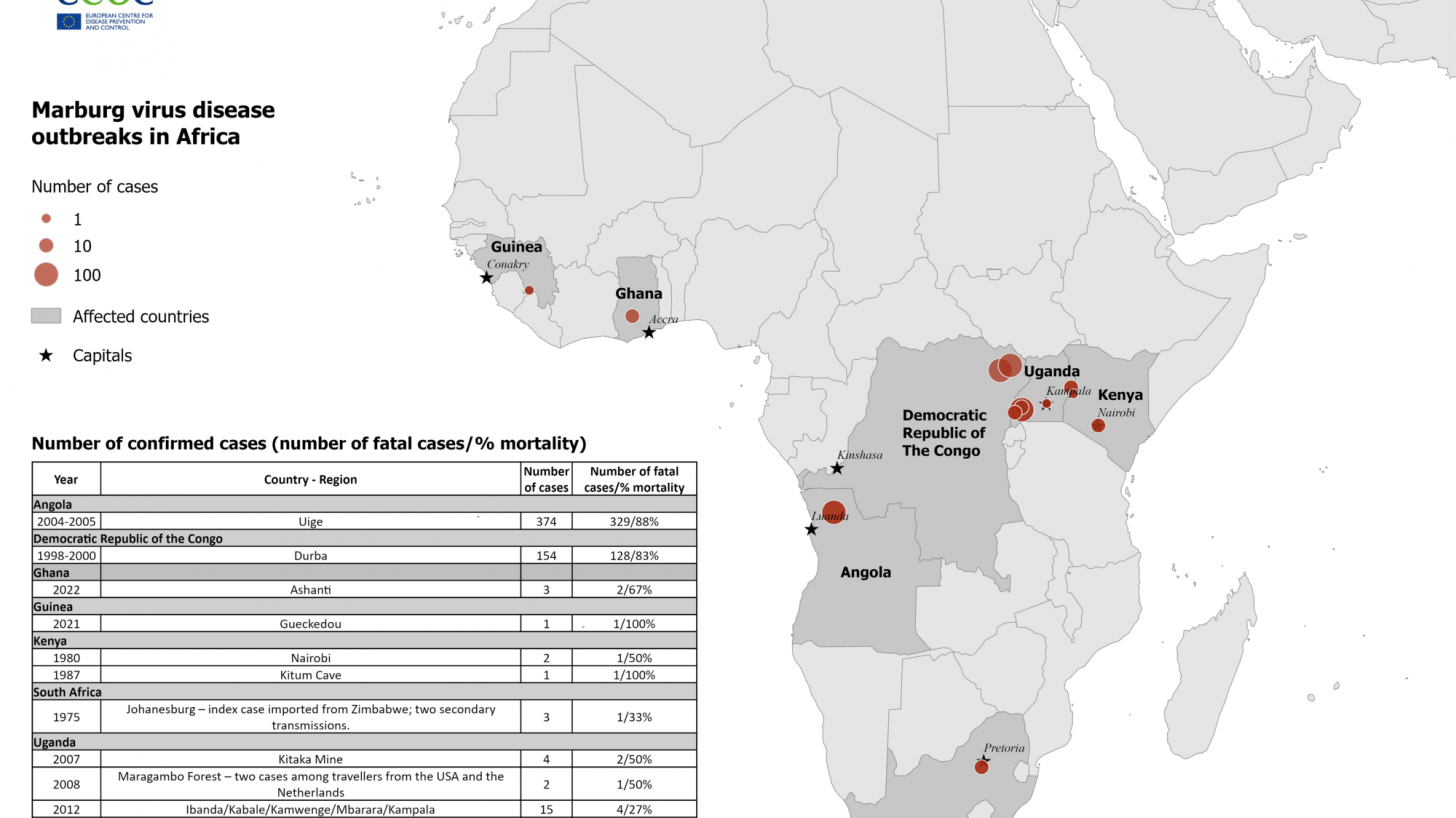
The European Centre for Disease Prevention and Control (ECDC) recently confirmed Marburg virus disease (MVD), formerly known as Marburg hemorrhagic fever, is a severe disease in humans caused by Marburg marburgvirus (MARV).
In 1967, two outbreaks co-occurred in Marburg, Germany, and Belgrade, Serbia, among laboratory workers working with tissues of African green monkeys imported from Uganda and medical personnel who cared for the laboratory workers.
Nine people of the 37 cases died, with some spreading through household or nosocomial contact.
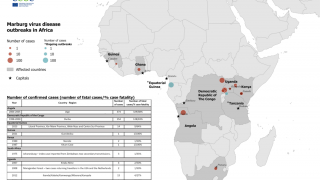
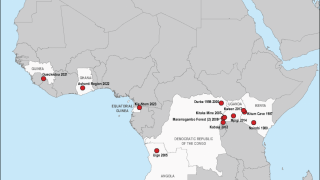
To date, all recorded MVD outbreaks have originated in Africa.
Nearly 600 Marburg cases have been reported in outbreaks in Uganda, the Democratic Republic of the Congo (DRC), and Angola.
The MVD outbreak in Durba, DRC (1998–2000) occurred among gold miners, with a case fatality rate of 83%, reported the ECDC.
Although MVD is uncommon, MARV can potentially cause epidemics with significant case fatality rates.
MVD is not an airborne disease and is considered not contagious before symptoms appear.
Direct contact with the blood and other body fluids of infected people and animals or indirect contact with contaminated surfaces and materials like clothing, bedding, and medical equipment is required for MARV transmission.
This vaccine development project will be funded with federal funds from the U.S. Department of Health and Human Services (HHS); Administration for Strategic Preparedness and Response; Biomedical Advanced Research and Development Authority, under contract number 75A50123C00010. BARDA, part of the HHS’ Administration for Strategic Preparedness and Response, is based in Washington, DC.
In addition to Ebola and Marburg concerns, the U.S. Centers for Disease Control and Prevention recently issued travel alerts for various disease outbreaks in Uganda, such as polio, measles, and yellow fever.
Our Trust Standards: Medical Advisory Committee