$35.7 Million Awarded For Marburg Virus Vaccine Development
The Defense Threat Reduction Agency (DTRA) of the U.S. Department of Defense (DoD) announced IAVI has been awarded $35.7 million dollars to develop a recombinant vesicular stomatitis virus (VSV) vector Marburg virus vaccine candidate, called rVSVΔG-MARV-GP.
The Marburg virus vaccine candidate, licensed by IAVI from the Public Health Agency of Canada, demonstrated strong protection from the deadly disease in non-human primate studies.
This is important news since the Marburg virus is a public health threat that has a high case fatality rate, and it is a potential bioterrorism threat.
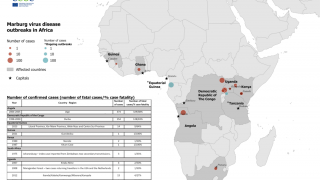
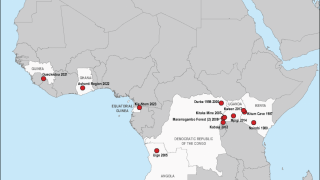
Marburg hemorrhagic fever is a very rare human disease. However, when it occurs, it has the potential to spread to other people.
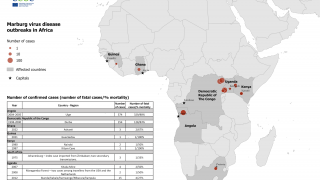
The Marburg virus was first recognized in 1967, when outbreaks of hemorrhagic fever occurred simultaneously in laboratories in Marburg and Frankfurt, Germany and in Serbia, says the US Centers for Disease Control and Prevention (CDC).
The CDC has classified the Marburg virus as a Category A bioterrorism threat, which indicates a high-priority agent that poses a risk to national security.
The reservoir host of the Marburg virus is the African fruit bat, Rousettus aegyptiacus. Fruit bats infected with Marburg do not show obvious signs of illness.
During 2018, the World Health Organization initiated a Blueprint list of priority diseases to accelerate Research & Development efforts for pathogens that bear a particularly high potential to cause epidemics while no or limited specific countermeasures are available and may, therefore, cause future public health emergencies.
The WHO’s Blueprint list includes the Marburg virus.
This DTRA award builds on IAVI’s expertise in VSV vector technology that has developed a VSV HIV vaccine candidate and VSV Lassa fever vaccine candidate, which are in preclinical development.
Mark Feinberg, M.D., Ph.D., president and CEO of IAVI, said, “IAVI looks forward to applying more than a decade of our experience in viral vector vaccines to hasten the development of this viral hemorrhagic fever vaccine candidate, and we are grateful to the U.S. Department of Defense for its support of this critical work.”
The Marburg virus vaccine candidate rVSVΔG-MARV-GP is based on the same VSV platform as Merck’s Ebola Zaire virus vaccine candidate that was tested during the 2014-2016 Ebola outbreak and is currently being used in the Ebola outbreak in the Democratic Republic of the Congo.
Recent Ebola news
- Merck’s Ebola Zaire Vaccine Approved in Europe
- USAID Funding Reaches $266 Million To Stop Ongoing Ebola Outbreak
As a vaccine vector, VSV delivers a gene from the target pathogen designed to lead to a long-lasting immune response in the recipient.
Data from an efficacy trial in Guinea of the Ebola Zaire virus vaccine candidate indicates that vaccine efficacy is as high as 100 percent.
Much of the preclinical work on the Marburg virus vaccine candidate will be performed by scientists at IAVI’s Vaccine Design and Development Laboratory (DDL) in Brooklyn, New York, which is home to IAVI’s VSV vaccine preclinical development.
In addition, critical work to validate the key assays needed to measure immune responses in clinical trials will be done at the IAVI Human Immunology Laboratory, based at Imperial College London.
IAVI’s research partners in this vaccine program are investigators at the Center for Global Infectious Disease Research; La Jolla Institute for Immunology; Ragon Institute of MIT, MGH, and Harvard; Tulane University; and University of Texas Medical Branch. The manufacturing partner is Batavia Biosciences. Clinical research partners in the U.S. and Africa are George Washington University, KAVI-Institute of Clinical Research (Kenya), Kenema Government Hospital (Sierra Leone), MRC/UVRI and LSHTM Uganda Research Unit, National Public Health Institute of Liberia, and Projet San Francisco (Rwanda). Merck & Co., Inc., is a scientific advisor.
The Defense Threat Reduction Agency enables the Department of Defense, the United States Government, and international partners to counter and deter weapons of mass destruction (WMD) and improvised threat networks.
As the DoD’s research and development leader focused on WMD and improvised threats, DTRA facilitates innovation through combining traditional research with unconventional means to develop and quickly field solutions to the most complex, deadly and urgent threats facing the United States and the rest of the world.
DTRA has over 2,000 uniformed military personnel and DoD civilians working on every continent except Antarctica.
Marburg Vaccine news published by Precision Vaccinations
Our Trust Standards: Medical Advisory Committee