First-in-Human Marburg Vaccine Study Shows Promise
The peer-review journal Lancet recently published a study that found a vaccine candidate targeting the Marburg virus (MARV) was safe and induced an immune response in a small, first-in-human clinical trial.
The vaccine known as cAd3-Marburg vaccine, developed by researchers at the U.S. National Institute of Allergy and Infectious Diseases (NIAID), could become an essential tool if approved to respond to future Marburg virus outbreaks.
This vaccine uses a modified chimpanzee adenovirus called cAd3, which can no longer replicate or infect cells.
Instead, it displays a glycoprotein found on the surface of MARV to induce immune responses against the virus.
The cAd3 vaccine platform demonstrated a good safety profile in prior clinical trials when used in Ebola virus and investigational Sudan virus vaccines.
This study enrolled 40 healthy adult volunteers at the Walter Reed Army Institute of Research Clinical Trials Center in Silver Spring, Maryland. They received a single dose of either a low dose of the vaccine (1x1010 particle units) or a higher dose (1x1011 particle units).
For safety, the volunteers were enrolled in a dose-escalation plan. First, three participants received the lower dose.
Then, when they did not exhibit severe adverse reactions after the first seven days, the trial enrolled the remaining 17 volunteers.
The same procedure was also used for the higher-dose group.
Volunteers were monitored for adverse reactions to the investigational vaccine and evaluated at regular intervals for 48 weeks to track their immune responses.
The trial’s safety results were encouraging, as there were no serious adverse events, and the experimental vaccine was well-tolerated.
One higher-dose group participant developed a fever following vaccination, which resolved by the following day.
In addition, the investigational vaccine appeared to induce strong, long-lasting immunity to the MARV glycoprotein: 95% of participants in the trial exhibited a robust antibody response after vaccination, and 70% maintained that response for more than 48 weeks.
There are plans to conduct clinical trials for the cAd3-Marburg vaccine in Ghana, Kenya, Uganda, and the U.S.
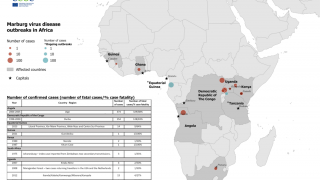
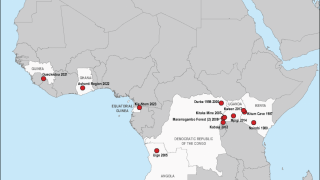
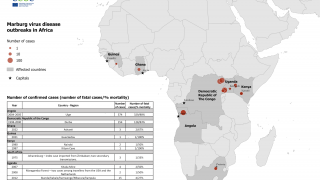
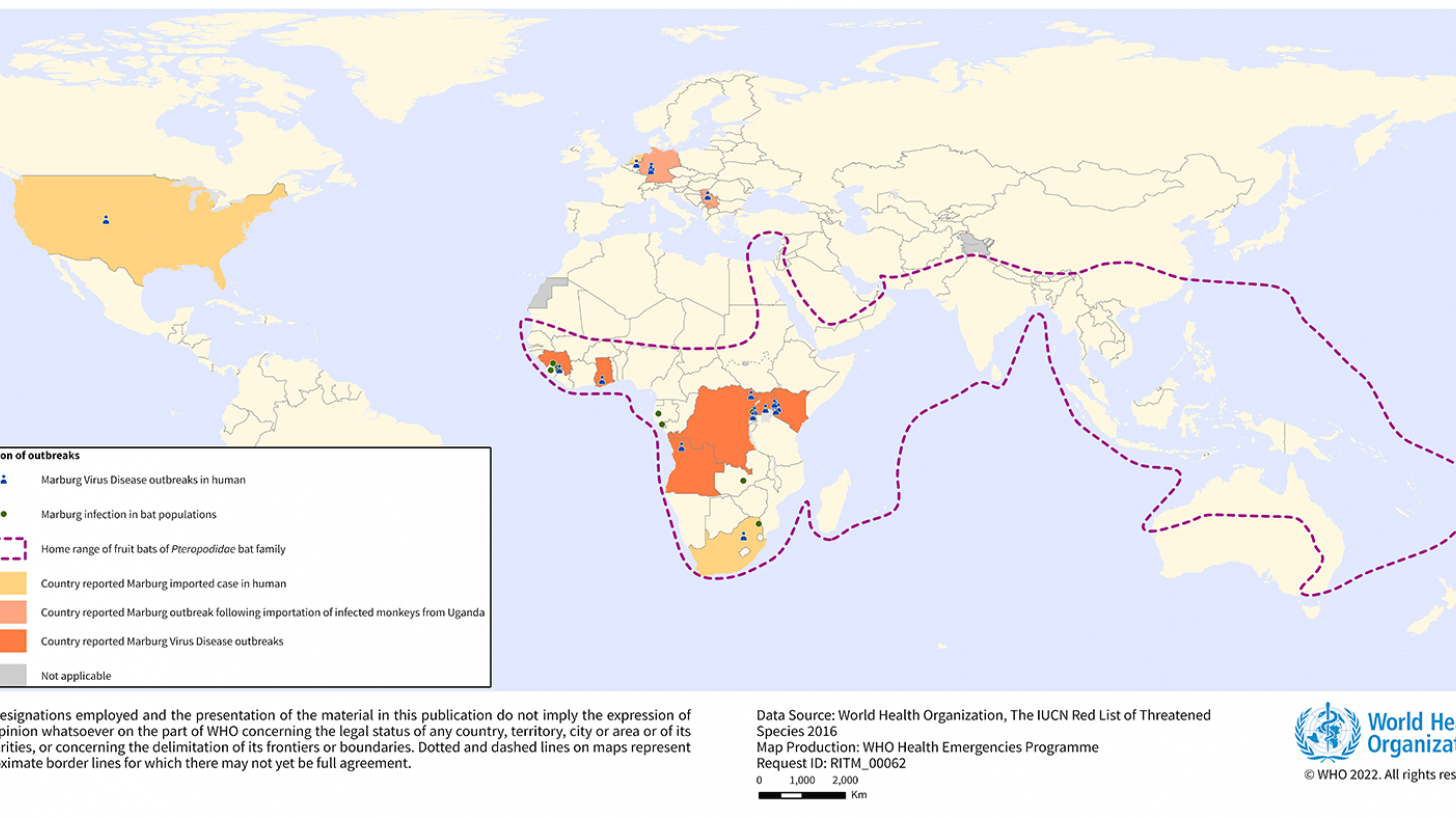
MARV, a filovirus in the same family as the Ebola virus, causes a rapidly progressive febrile illness that leads to shock and death in many infected individuals, says the U.S. CDC.
Marburg virus was first recognized in 1967 when hemorrhagic fever outbreaks co-occurred in laboratories in Germany and Serbia.
MVD is caused by the MARV, a genetically unique zoonotic RNA virus of the filovirus family.
In areas of Africa where a vaccine for Marburg is most needed, a single-dose vaccine that could protect recipients over a long period would be a crucial part of quelling outbreaks.
As of January 30, 2023, no vaccines or specific therapies are U.S. Food and Drug Administration approved for MARV disease.
The NIAID conducts and supports research throughout the U.S. and worldwide to study the causes of infectious and immune-mediated diseases and to develop better means of preventing, diagnosing, and treating these illnesses. News releases, fact sheets, and other NIAID-related materials are available on the NIAID website.
Our Trust Standards: Medical Advisory Committee