Novel Tuberculosis Drug Regimens Tested
Despite introducing new medicines, treatment remains a long, complex, and demanding process for people diagnosed with tuberculosis (TB). And the most used drug regimen for the treatment requires people to take multiple drugs for at least six months.
The World Health Organization (WHO) estimates that nearly half of TB-affected households face catastrophic costs associated with TB treatment.
The Project to Accelerate New Treatments for Tuberculosis (PAN-TB) collaboration announced on August 9, 2023, launches with a phase 2b/c clinical trial sponsored by the Bill & Melinda Gates Medical Research Institute (Gates MRI). The goal is to identify a candidate regimen suitable for phase 3 development.
The trial will evaluate whether novel regimens that combine registered products and new chemical entities have the potential to effectively treat drug-sensitive TB (DS-TB) and inform the development of a "pan-TB" regimen capable of treating all forms of active pulmonary TB.
The regimens under evaluation are designed to explore shorter treatment durations than existing drug regimens without the need for accompanying drug-resistance testing for individuals.
The collaboration is evaluating two novel drug regimens comprising five antibacterial agents—bedaquiline, delamanid, pretomanid, quabodepistat (formerly known as OPC-167832), and sutezolid:
DBQS – delamanid, bedaquiline, quabodepistat and sutezolid
PBQS – pretomanid, bedaquiline, quabodepistat and sutezolid
To support participants during their treatment, the trial integrates a Stop Treatment and Watch (STrAW) Concilium, a group of expert clinical consultants that will evaluate trial participants' treatment response, blinded to the specific regimen they receive, and advise trial investigators on patient management.
Emilio Emini, Ph.D., CEO of the Bill & Melinda Gates Medical Research Institute, said in a press release, "The Bill & Melinda Gates Medical Research Institute is committed to developing biomedical interventions that address global health concerns for those in the greatest need."
"The team at the Gates MRI looks forward to working with our partners across the PAN-TB collaboration to evaluate the potential of these novel TB treatment regimens, in the hope that it may offer a potentially easier pathway for all people diagnosed with pulmonary TB by offering well-tolerated, shorter and simpler treatment options."
The PAN-TB collaboration leverages members' collective assets, resources, and scientific expertise to identify and evaluate new drug regimens with an acceptable safety profile that have the potential to treat both drug-sensitive and drug-resistant TB and are well-tolerated, shorter in duration, and simpler to use than existing options.
The PAN-TB collaboration plans to work closely and transparently with the European Regimen Accelerator for Tuberculosis (ERA4TB), which was launched in January 2020.
New molecular entities identified by ERA4TB that show promise in initial human studies could be incorporated into the PAN-TB collaboration's later-stage clinical research.
Several organizations, including Evotec, GSK, TB Alliance, and Janssen Pharmaceutica NV, are members of both projects, which will help ensure coordination across collaborations toward advancing TB drug and regimen development.
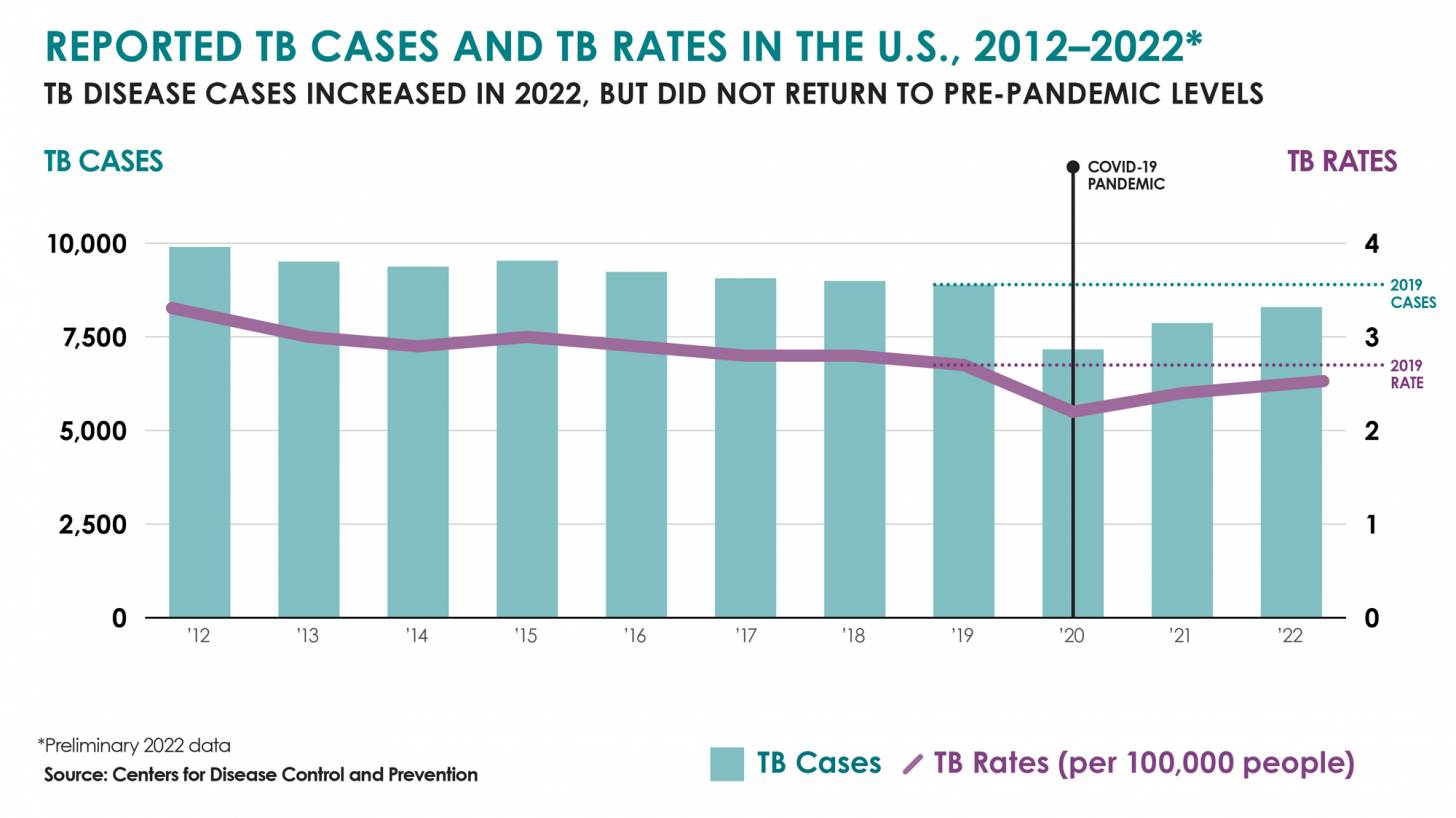
While most TB cases are reported in other countries, preliminary TB data released by the U.S. CDC show that the number of U.S. TB disease cases increased by 5% in 2022 to 8,300 cases.
In addition, the CDC reported 202 cases of TB in children in 2022, an increase from 160 cases in 2021.
California, New York, and Texas are leading states reporting TB cases.
TB is a vaccine-preventable disease, with Bacillus Calmette–Guérin vaccination, says the WHO. While TB vaccines have been deployed for about 100 years, there are not 100% effective, and innovative drug regimens are needed in 2023.
Our Trust Standards: Medical Advisory Committee
























