Is the U.S. CDC Expecting a Tuberculosis Outbreak
The U.S. Centers for Disease Control and Prevention (CDC) Division of Global Migration Health published enhanced instructions that define panel physicians' specific responsibilities in testing for and treating infectious tuberculosis (TB) disease among applicants from overseas.
As of January 16, 2024, the new TB screening summary from the CDC is as follows:
- Applicants in all countries who are ≥15 years old must have a chest x-ray.
- Applicants 2 years of age and older examined in countries with a World Health Organization (WHO)-estimated tuberculosis incidence rate of ≥20 cases per 100,000 population must have an interferon-gamma release assay (IGRA).
- Panel physicians may advise applicants for whom testing is clinically indicated about HIV testing.
These instructions are specific to an immigration medical examination.
The molecular testing requirement, IGRA testing for adults, and changes to drug susceptibility testing defined in these instructions go into effect no later than October 1, 2024.
All other instructions components go into effect on January 24, 2024.
While the United States has traditionally reported very few TB outbreaks, this vaccine-preventable disease has impacted 192 countries and areas.
According to the WHO 2023 Global TB Report, an estimated 10.6 million people fell sick with TB in 2022, and 1.3 million people died from TB.
In 2022, most people who developed TB lived in Southeast Asia (46%), Africa (23%), and the Western Pacific (18%).
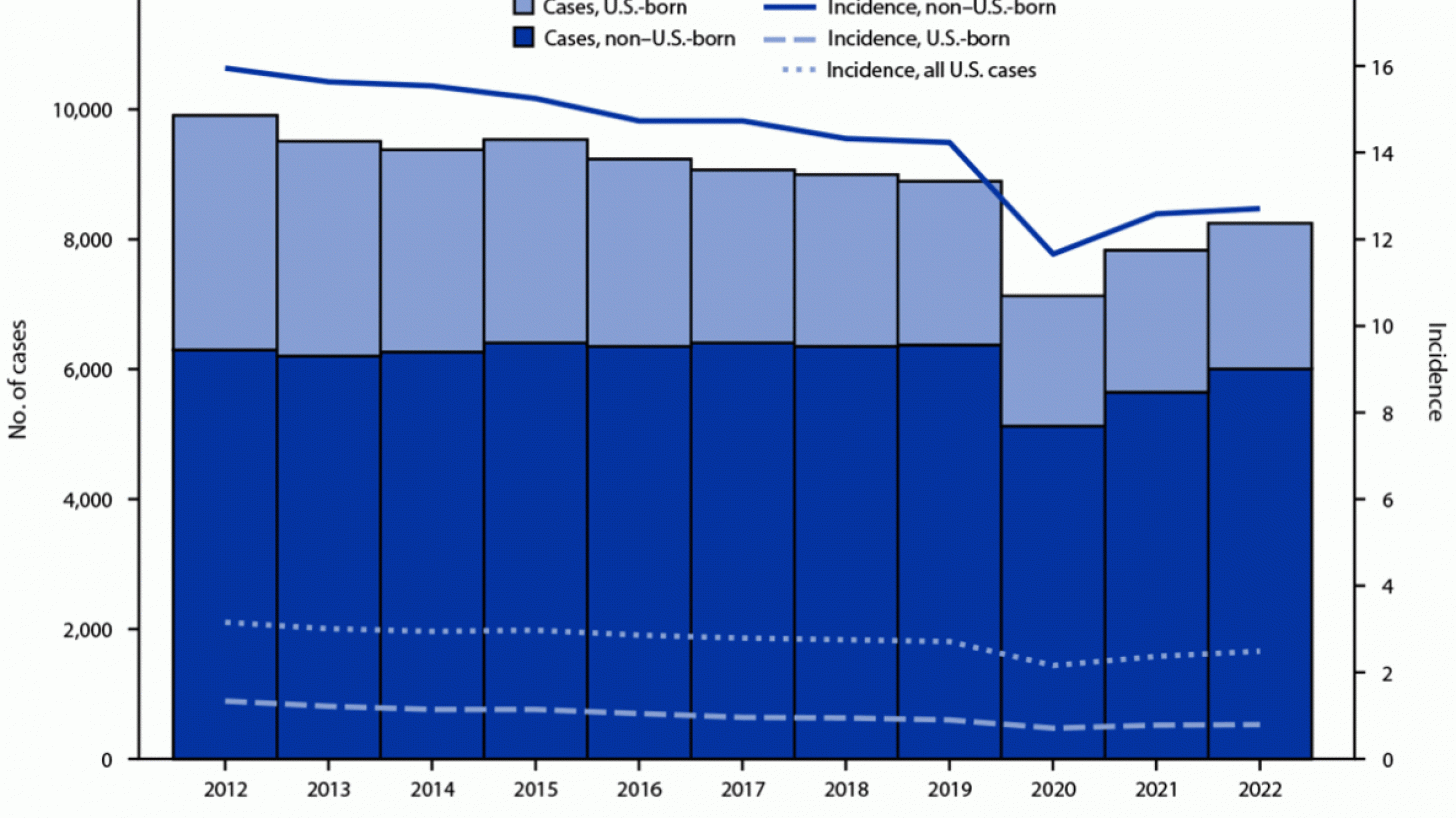
In the United States, the CDC reported on November 15, 2023, that there were 8,331 TB cases in 2022, an increase of just 5% from 2021.
However, the CDC says about 13 million people living in the U.S. may have a latent TB infection.
Furthermore, 73% of TB cases in persons for whom birth origin was known, cases occurred among non–U.S.-born persons.
TB can often be prevented with a 100-year-old vaccine.
Blessina Kumar, CEO of the Global Coalition of TB Advocates, confirmed that 16 different Bacille Calmette-Guerin (BCG) vaccines are available in 2024.
And various BCG vaccine candidates are under development in response to about 14 BCG sub-strains that have evolved.
In the U.S., Merck's TICE® BCG vaccine is available under specific requirements at most health departments but is not generally available at health clinics or pharmacies.
Our Trust Standards: Medical Advisory Committee
























