Lyme Disease Testing May Depend on Autoantibody Biomarkers

Researchers at Massachusetts-based Tufts University School of Medicine recently announced they identified a testing mechanism that detects a type of antibody, infected individuals produce, against a substance the Lyme bacteria acquires from the host to grow.
These researchers stated on March 15, 2022, they believe tests to detect auto-antibodies, which are antibodies that mistakenly target and react with a person's tissues or organs, could empower clinicians with a way to diagnose Lyme disease sooner.
And to know whether treatment with treatment antibiotics is working and identify which patients have been reinfected.
Authors of the study, published by the Journal of Clinical Investigation, are Peter Gwynne, Luke Clendenen, and Linden Hu of the school’s Department of Molecular Biology and Microbiology, and colleagues at the National Institute of Allergy and Infectious Diseases at the National Institutes of Health.
Testing to detect Lyme disease exists, but it has limitations, says Gwynne, the study's lead author and research scientist at Tufts School of Medicine.
“Traditional Lyme tests can stay positive for prolonged periods after treatment – years or even a lifetime,” he commented in a related press release.
“As a result, for some individuals suffering from symptoms that resemble long-term Lyme disease infection, clinicians are never sure whether the patient has persistent Lyme disease, was cured and then reinfected, or was cured and is suffering from something else.”
“We started this current work to learn how Borrelia burgdorferi acquires key nutrients, like fats, for growth,” says Gwynne.
“The Lyme bacteria, despite being a very successful pathogen, is much more dependent than other bacteria on acquiring nutrients from its environment.”
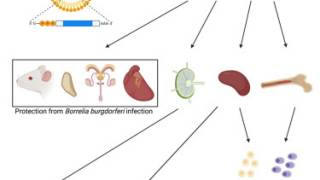
“In our research, we found that the organism takes fats called phospholipids directly from its surroundings in the host and puts them on its surface,” added Hu, the Vice Dean of Research at the school and Paul and Elaine Chervinsky Professor of Immunology.
“That finding led us to see if the bacteria's direct use of a host fat might lead the immune system to recognize it as a foreign substance and create antibodies to it.”
The scientists discovered that both animals and patients infected with the Lyme bacterium developed autoantibodies to multiple phospholipids.
Because autoantibodies can damage the host, these autoantibodies are tightly regulated and tend to disappear quickly once the stimulating factor is removed.
“The antibodies also seem to develop much more quickly than traditional antibodies to the Lyme bacteria, likely because your body has previously created these autoantibodies and downregulated them,” says Hu.
While current testing makes it difficult to diagnose reinfection or successful treatment, “the antiphospholipid autoantibodies—because of their quick increase and quick resolution with treatment—can fill these gaps as a novel additional test,” Gwynne says.
“They may make it possible to tell whether a treatment has eradicated the Lyme disease bacteria. And they therefore also make it possible to tell if a patient with a prior infection now has a new infection.”
Gwynne and Hu have a provisional patent pending describing the use of antiphospholipid antibodies in diagnosing Lyme disease. They hope that if their discovery is borne out by further research, a diagnostic company could begin the development of a commercially available version of their test within a couple of years.
Lyme disease was identified five decades ago along the Connecticut coast and spread across New England and the mid-Atlantic region, affecting almost 500,000 people in the U.S. every year.
Caused by a bite from an infected tick, it frequently goes undetected unless a person notices the telltale rash that forms around the bite.
Lyme disease can lead to debilitating long-term complications, including arthritis, fatigue, mental impairment, and attacks on the heart and brain tissue in the most severe cases.
Caused by the bacterium Borrelia burgdorferi, Lyme disease can often be treated with antibiotics.
An important question, which was not examined in the current paper, is whether these autoantibodies may identify a subset of patients who will develop persistent Lyme disease symptoms after treatment.
Up to 20% of patients can develop persistent symptoms after Lyme disease. Diagnosis of these patients is currently only by clinical symptoms, making it likely that patients with different causes of their symptoms are grouped together.
And treatment trials in patients with persistent Lyme disease are unlikely to benefit if that occurs.
“Antiphospholipid antibodies are commonly seen in autoimmune diseases like lupus and are associated with blood clots and persistent inflammation that causes other disease conditions,” says Hu.
“Many of the persistent symptoms in patients who continue to have symptoms after being diagnosed with Lyme disease are similar to those autoimmune diseases.”
“If there ends up being a link between having persistent Lyme symptoms and these autoantibodies, this would be the first test that could be used to distinguish a group of patients who have persistent Lyme disease,” he says.
“It would allow us to test specific new therapies targeted to a defined mechanism.”
Currently, the U.S. FDA has not approved a Lyme disease vaccine. However, there are Lyme disease vaccine candidates in late-stage studies.
An example is Valneva SE’s Lyme Disease vaccine candidate VLA15, a multivalent recombinant protein vaccine targeting the outer surface protein A of Borrelia.
Valneva and its development partner Pfizer Inc. announced on February 4, 2022, a three-dose primary series vaccination schedule in a planned Phase 3 clinical trial is expected to be initiated in 2022, subject to regulatory approval.
PrecisionVaccinations publishes fact-checked research-based vaccine news.
Note: The press release and study results were edited for clarity and curated for mobile readers.
Our Trust Standards: Medical Advisory Committee