153 RSV Vaccination Errors Reported
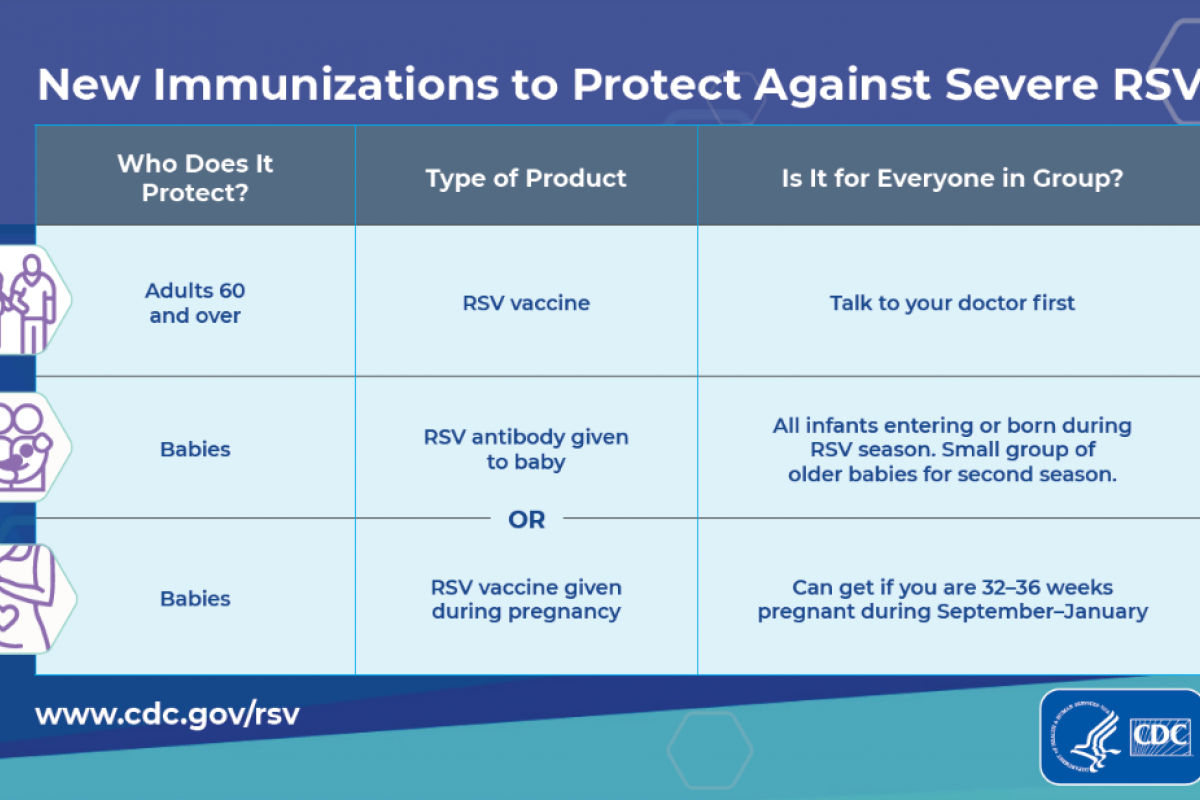
Since the approval of Respiratory Syncytial Virus (RSV) vaccines and an enhanced monoclonal antibody in 2023, the U.S. Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) have received reports of RSV vaccines being administered in error to young children and pregnant women.
A Clinician Outreach and Communication Activity COCA Now email issued today says the number of reports received by the Vaccine Adverse Event Reporting System (VAERS) as of January 17, 2024, suggests that these types of errors are uncommon in young children less than two years of age (25 reports) and pregnant women (128 reports).
The CDC stated this is a relatively small amount of errors compared to the estimated one million infants protected from RSV either through infant receipt of Beyfortus™ (nirsevimab) or by pregnant women being vaccinated.
According to the CDC, most of these administration error reports described no adverse event. When an adverse event was concurrently reported to VAERS, most reports were classified as nonserious.
The CDC, FDA, and other federal agencies said they continue to monitor the safety of RSV vaccines and reports of vaccine administration errors and will share information with the public as it becomes available.
On January 22, 2024, the CDC published Recommendations for Healthcare Providers who Have Administered Incorrect RSV Vaccine Products to Their Patients.
Our Trust Standards: Medical Advisory Committee























