Thermostable Bivalent Filovirus Vaccine Candidate May Protect Against Ebola and Marburg Viruses
The journal Vaccine recently published a manuscript entitled "Thermostable bivalent filovirus vaccine protects against severe and lethal Sudan ebolavirus and marburgvirus infection."
This publication describes the preclinical efficacy of a novel, single-vial, bivalent thermostabilized vaccine providing 100% protection in the most rigorous non-human primate challenge models against Sudan ebolavirus (SUDV) and Marburg marburgvirus (MARV) infections.
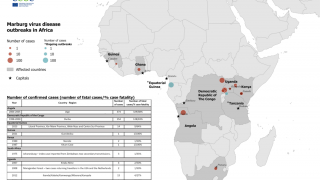
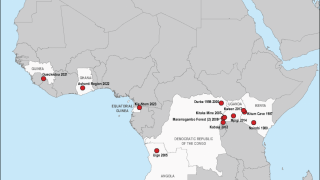
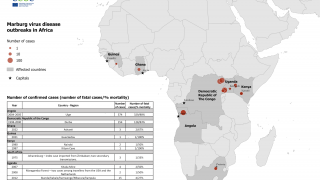
Recent outbreaks have occurred in Africa, with increased frequency in 2023.
There are currently no approved vaccines or therapeutics for either SUDV or MARV infections.
However, vaccines are available for Zaire ebolavirus (EBOV) infections in 2024, but they provide no protection against SUDV or MARV infection.
"Filoviruses such as EBOV, SUDV, and MARV are some of the most lethal viruses known, and they are endemic in areas of the world where the power supply and distribution network can be uncertain, says the World Health Organization.
A thermostabilized vaccine in a single vial format would significantly enhance any public health response to a new outbreak, at its source," stated Axel Lehrer, Ph.D., Associate Professor, Department of Tropical Medicine, Medical Microbiology and Pharmacology, University of Hawaiʻi at Mānoa, in a press release.
"Our work to date has demonstrated the feasibility of rapid and efficient manufacturing, as well as the ability to thermostabilize multiple antigens that can then be stored for extended times at temperatures exceeding 100 degrees Fahrenheit."
"The use of a bivalent vaccine has the potential to both prevent future infections with these pathogens and potentially mitigate future outbreak events, potentially using an accelerated dosing regimen."
The thermostabilized filovirus vaccine program continues to advance with the support of a National Institute of Health grant and a Small Business Innovation Research grant awarded to Soligenix, Inc.
Our Trust Standards: Medical Advisory Committee