Which Country Needs a Chikungunya Vaccine
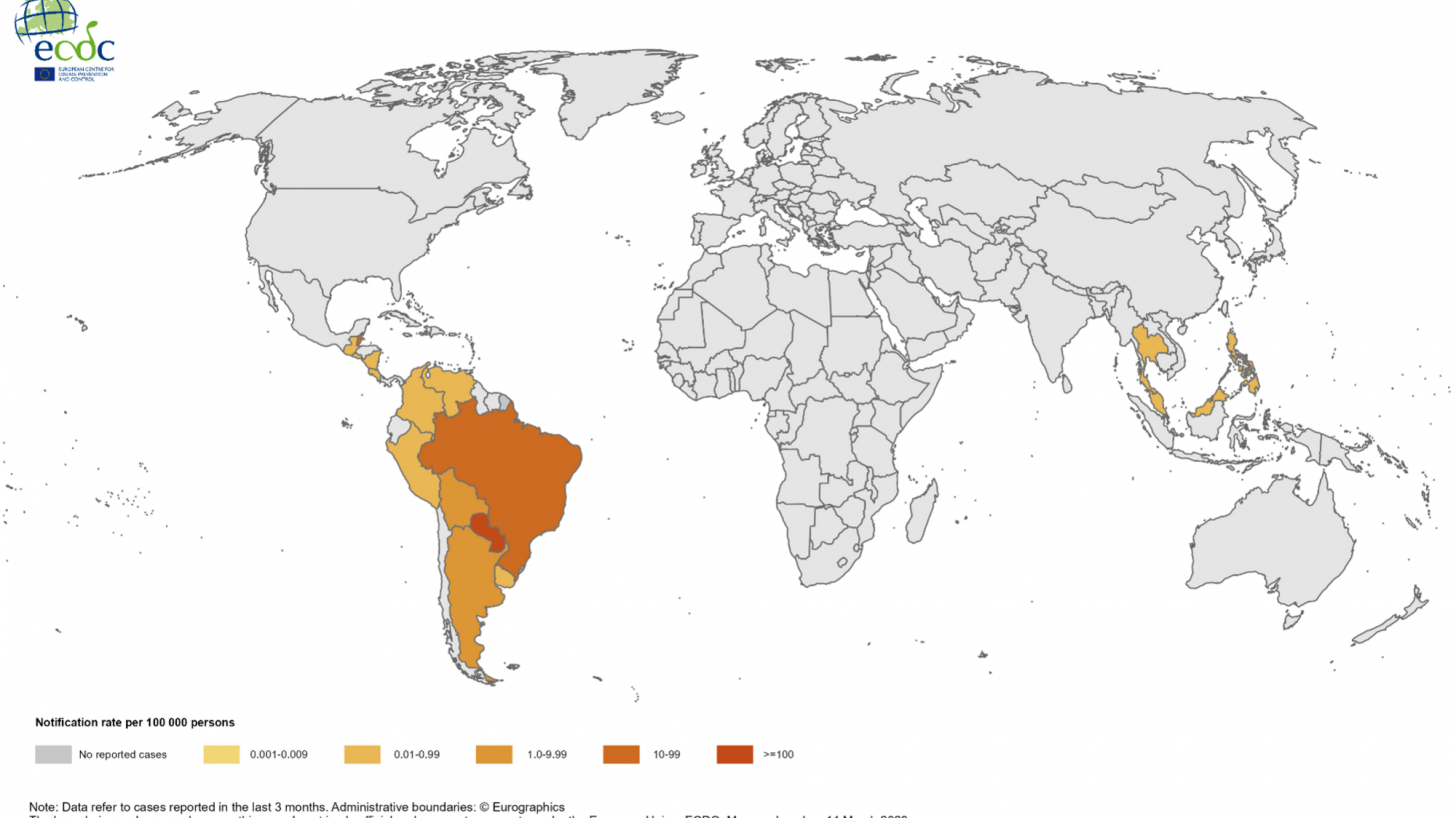
During the first months of 2023, significant chikungunya fever outbreaks were recorded in South America, Central America, and Asia.
On June 10, the Pan American Health Organization (PAHO) published an Epidemiological Update regarding chikungunya outbreaks in the Region of the Americas.
Early this year, the European Centre for Disease Prevention and Control published a worldwide overview of chikungunya outbreaks.
Both of these reports highlight an outbreak in Paraguay.
As of March 2023, 82,240 chikungunya cases and 43 deaths had been reported in Paraguay.
Other countries reporting outbreaks include, but are not limited to, the following:
Argentina: In 2023, and as of February 25, 655 cases, including 161 confirmed cases and no deaths, had been reported.
Belize: In 2023 and as of February 4, 46 cases.
Bolivia: In 2023 and as of February 11, 300 cases.
Brazil: In 2023 and as of February 25, 30,386 cases and no deaths had been reported.
Guatemala: In 2023 and as of February 11, 40 cases.
Malaysia: In 2023 and as of February 18, 94 confirmed cases.
Peru: In 2023 and as of March 4, 97 cases.
Philippines: In 2023 and as of February 18, 29 confirmed cases.
Thailand: In 2023 and as of February 25, 259 confirmed cases.
The PAHO / World Health Organization recommends that Member States review and adjust their preparedness and response plans to face possible outbreaks of arboviral diseases to avoid deaths and complications from these diseases before the start of the high transmission season.
The Chikungunya virus is spread to people by the bite of an infected mosquito. The most common symptoms of infection are fever and joint pain, says the U.S. CDC.
And in the U.S., there were 36 travel-associated cases reported in 2021.
While the outbreaks are very concerning, an innovative vaccine could soon be authorized to protect people.
Valneva SE announced on June 13, 2023, the phase 3 clinical trial data for its single-shot chikungunya vaccine candidate, VLA1553.
This innovative vaccine demonstrated a seroresponse rate of 98.9% in participants 28 days after receiving the single administration, and 96% of participants maintained seroresponse six months after vaccination.
Other chikungunya vaccine news is posted by Vax-Before-Travel.
Our Trust Standards: Medical Advisory Committee
























