Chikungunya Outbreaks Accelerate and Turn Fatal
The Pan American Health Organization (PAHO) today published an Epidemiological Alert regarding chikungunya outbreaks in the Region of the Americas. The PAHO confirmed this mosquito-borne virus is expanding to new areas with an unusually high number of cases and related fatalities.
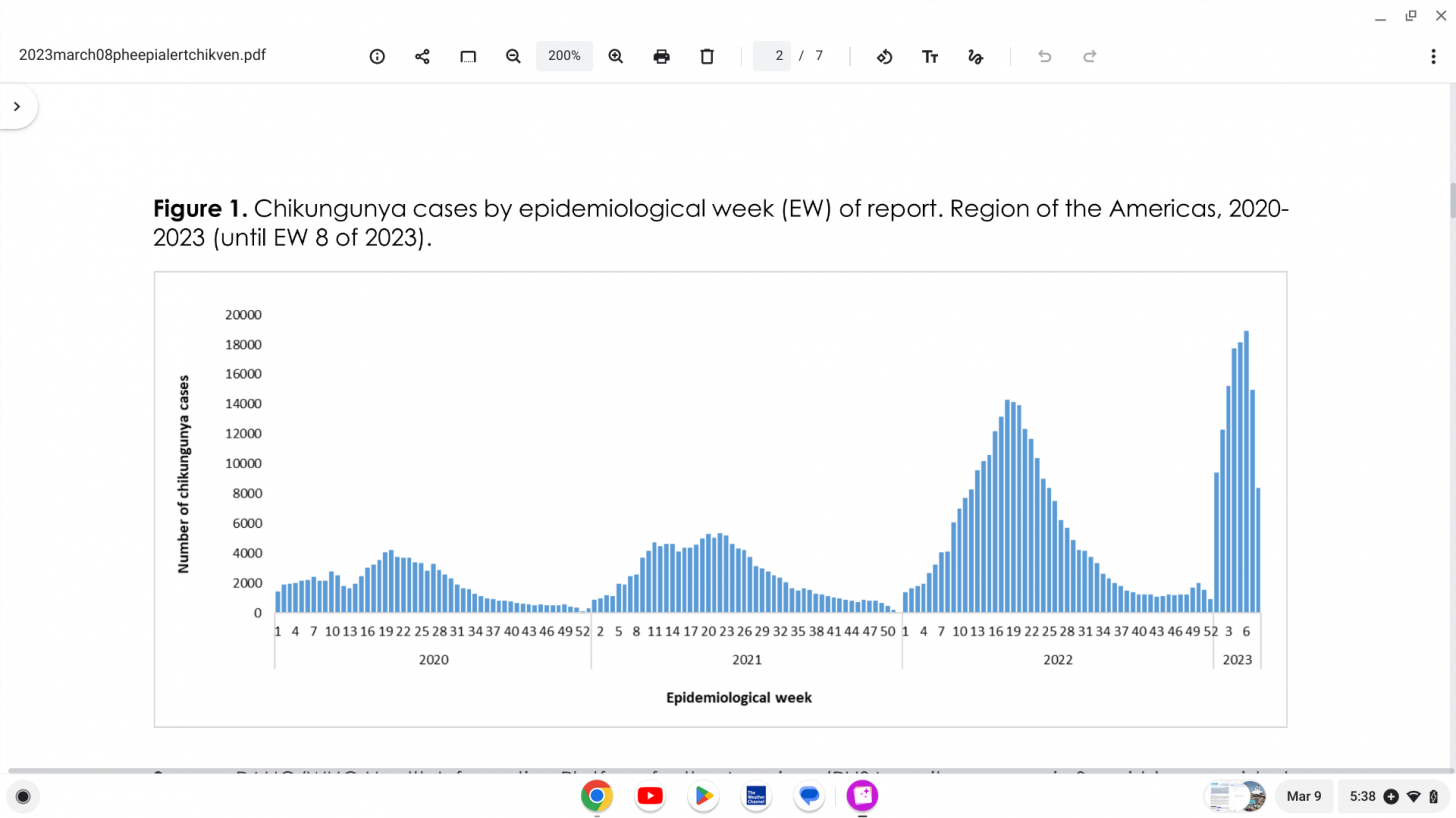
During the first eight epidemiological weeks of 2023, 115,539 cases and 33 fatalities due to chikungunya infection were reported.
The greatest incidence rates were observed in Paraguay (1,128 cases per 100,000 pop.) and Brazil (14.2 cases per 100,000 pop.).
The PAHO stated on March 8, 2023, these increases exceed previous years' surveillance.
Additionally, there are simultaneous circulations of other arboviral diseases, such as dengue.
These diseases are transmitted by the same vectors, Aedes aegypti (most prevalent) and Aedes albopictus.
The PAHO says it is essential for the entire Southern Hemisphere to be highly vigilant and prepared to intensify health services' prevention, control, and preparedness actions in the face of any increase in cases of arboviruses, especially chikungunya.
Chikungunya outbreaks are occurring at an accelerated pace as of March 2023.
Furthermore, the PAHO reiterates that Member States intensify actions to prepare healthcare services, including the diagnosis and proper management of cases; and to strengthen prevention and vector control measures to reduce the impact of this and other arboviral diseases.
While there are two approved dengue vaccines available in various countries, the U.S. Food and Drug Administration (FDA) and the European Medicines Agency have not approved a vaccine to prevent the impact of chikungunya infection.
During the recent U.S. Centers for Disease Control and Prevention Advisory Committee meeting on February 23, 2023, the panel of vaccine experts reviewed the VLA1553 monovalent, single-dose, live-attenuated vaccine candidate.
The good news is that France-based Valneva SE's VLA1553 has been assigned an FDA Prescription Drug User Fee Act review goal date at the end of August 2023.
Until a vaccine is approved, the PAHO suggests avoiding mosquito bites is the best prevention against chikungunya infection.
Our Trust Standards: Medical Advisory Committee
























