U.S. Unprepared for Next Smallpox Outbreak

A recent report suggests that the United States needs to take action to improve its capability to handle smallpox and related outbreaks.
The report, published by the National Academies of Sciences, Engineering, and Medicine on March 26, 2024, highlights the need to enhance diagnostics, vaccines, and therapeutics that could be used in the event of an outbreak.
The report also notes that changes in population and advancements in gene editing and synthesis technologies have significantly increased the likelihood of a smallpox outbreak or attack.
In 2024, it is possible to engineer the variola virus, which causes smallpox, raising the possibility of accidental or intentional release.
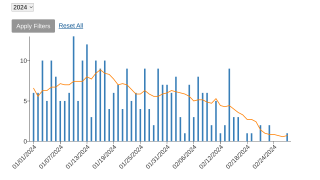
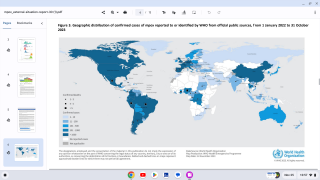
Furthermore, illnesses related to smallpox, such as mpox, Alaskapox, and cowpox, are increasingly found in humans, presenting the need for medical countermeasures that can detect, treat, and prevent these diseases.
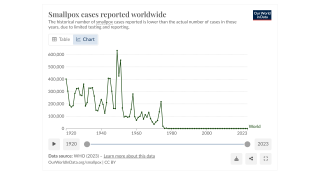
Before smallpox was eradicated in 1949, about 30% of infected people died, with many blinded or permanently scarred, says the U.S. CDC.
The report says that developing better diagnostics, vaccines, and therapeutics — also called medical countermeasures — would improve the nation's ability to respond to a smallpox outbreak or attack.
It also calls for strengthening the systems and policies allowing public health and healthcare systems to act quickly and effectively, such as those that could support the rapid vaccine distribution.
The Committee wrote that safer vaccines that can be used across different populations and are available as a single dose would support a faster and more effective response to a smallpox outbreak.
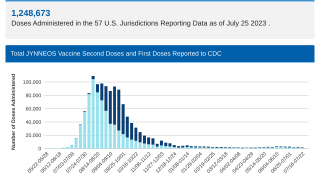
Currently, the U.S. FDA has approved two vaccines to target smallpox: ACAM2000® and JYNNEOS®.
The U.S. Department of Health and Human Services awarded a contract in September 2019 valued at approximately $2 billion over ten years for the continued supply of ACAM2000 into the U.S. Strategic National Stockpile.
Developing new smallpox vaccines that use a multi-vaccine platform—which uses common vaccine vectors, manufacturing ingredients, and processes—would improve the capacity for rapid production and reduce the need for stockpiling.
The small number of manufacturers capable of producing smallpox medical countermeasures is a specific vulnerability, and there is currently insufficient capacity to scale production in the event of a large outbreak or attack.
In the case of an international smallpox emergency, U.S. readiness and response capabilities will be significantly affected by the ability of other countries to detect and contain smallpox transmission.
Therefore, supporting international capacity and the ability to access smallpox medical countermeasures will improve U.S. biosecurity, concluded this Committee.
This study, undertaken by the Committee on the Current State of Research, Development, and Stockpiling of Smallpox Medical Countermeasures, was sponsored by the Administration for Strategic Preparedness and Response.
Our Trust Standards: Medical Advisory Committee