Third Mpox Vaccination Not Recommended
While numerous mpox vaccine breakthrough cases have been reported in the United States and other countries in 2023, many patients and healthcare providers have sought clinical guidance regarding how many vaccinations are appropriate.
During the U.S. Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) meeting on June 23, 2023, Agam Rao, MD CAPT, U.S. Public Health Service, presented considerations for long-term protection against mpox.
Dr. Rao's summary stated the mpox workgroup prefers no CDC recommendation for a third JYNNEOS® (MVA-BN) vaccination at this time.
This CDC advice includes persons with advanced HIV or other severe immunocompromise.
Additionally, the CDC emphasized encouraging 2-dose vaccinations among persons who do not have immunity and optimizing immune function (e.g., with HIV antiretrovirals), ideally before mpox exposure.
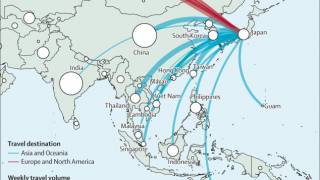
As of mid-June, mpox breakthrough cases have been reported in Chicago, Denver, New Jersey, and New York in the U.S.
And internationally, JYNNEOS breakthrough cases have been reported in Japan, France, South Korea, and the United Kingdom.
The CDC's Morbidity and Mortality Weekly Report regarding the Chicago cases published on June 23, 2023, wrote although the cause of this cluster has not yet been determined, leading hypotheses include a potentially high number of sexual exposures in a network with many vaccinated persons, decreased vaccine effectiveness due to waning of humoral immunity, or vaccine mishandling or administration errors.
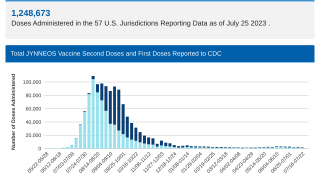
And persons eligible for vaccination, particularly those with advanced HIV and other immunocompromising conditions, should receive two doses of JYNNEOS vaccine.
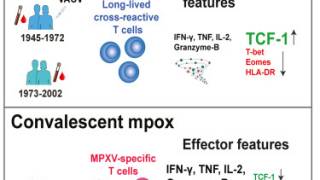
Additional research on the durability of JYNNEOS vaccine–induced immunity is needed.
Our Trust Standards: Medical Advisory Committee