Maximizing Mpox Antibodies: Natural Infection Vs. Vaccination

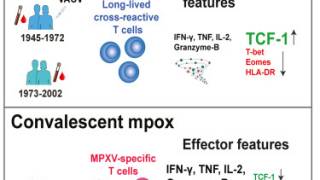
This new study compared the neutralizing antibody response after a mpox infection or Modified Vaccinia Ankara (MVA-BN, JYNNEOS®) vaccination.
Published by The Lancet Infectious Disease on October 11, 2023, these researchers concluded that people vaccinated with MVA-BN® frequently developed low or medium mpox neutralizing antibodies compared to infected individuals.
Furthermore, about 10% of vaccinated individuals showed no detectable neutralizing antibodies at six months, whereas every person with mpox infection developed antibodies.
And people living with HIV, when compared with people without HIV, developed more frequently low or medium-neutralizing antibody responses, which might be related to premature cellular senescence.
This study included individuals with mpox infection (May–Nov 2022) or who received MVA-BN vaccination (August–November 2022) at the Infectious Diseases Unit of San Raffaele Scientific Institute, Milan, Italy.
The plaque reduction neutralization test (PRNT) established mpox-neutralizing antibody titres six months after infection or first vaccination. Titres were grouped as low (L-mpox ≤1/10), medium (M-mpox 1/20–1/80), or high (H-mpox ≥1/160).
Multivariable multinomial logistic regression and classification tree analysis were applied.
Overall, 180 people were included: 95 with mpox infection and 85 vaccinated with MVA-BN. All were males, and 99% were men who had sex with men.
The median age was 37·2 years, and previous smallpox vaccination was reported by 10% of people; 36% were people living with HIV, and 4% were immunosuppressed for other causes.
Individuals who received MVA-BN vaccination had significantly lower concentrations of mpox neutralizing antibodies than those with mpox infection (p<0·0001).
The most common value of antibodies among those with infection was 1/80 (29 [31%]), and all had detectable neutralizing antibodies.
Among people who received MVA-BN vaccination, the most common value was 1/10 (29 [34%]), and 10 (12%) had no detectable antibodies (<1:10).
At multivariable regression, vaccinated people, rather than those with mpox infection, had a significantly higher risk of having L-mpox antibody titres (adjusted odds ratio [aOR] 319·84; 95% CI 43·44–2354·85; p<0·0001) and M-mpox titres (aOR 9·02; 95% CI 2·44–33·31; p<0·0001).
People living with HIV had a higher risk of having L-pox titres (aOR 3·99; 95% CI 1·01–15·75; p=0·050) but not M-pox (aOR 1·63, 95% CI 0·68–3·89; p=0·27), compared with those not living with HIV.
Older people had a lower risk of having L-mpox titres (aOR 0·92; 95% CI 0·85–1·00; p=0·060) or M-mpox (aOR 0·94; 95% CI 0·89–0·99; p=0·020).
People with or without immunodepression had a similar risk of L-mpox titres (aOR 0·40; 95% CI=0·02–6·66; p=0·52) or M-mpox (aOR 0·59, 95% CI 0·10–3·30; p=0·55).
The highest probability of H-mpox antibodies was among people with mpox infection aged at least 42 years (31 [17%] of 180, L-mpox 0, M-mpox 11 [35%] of 31, H-mpox 20 [65%] of 31).
The highest of L-mpox antibodies was among vaccinated people living with HIV (15 [8%] of 180, L-mpox ten [67%] of 15, M-mpox four [27%] of 15, H-mpox one [7%] of 15).
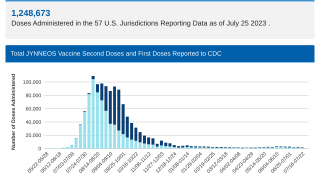
Although this study identified people with low or no detectable antibody titres, MVA-BN vaccination has been shown in real-world settings since May 2022 as a powerful tool for mpox prevention.
As the 2022 mpox outbreak spread worldwide, health leaders assumed vaccination with an approved smallpox vaccine (JYNNEOS) might provide some level of protection against the mpox virus.
The U.S. CDC Emerging Infectious Disease published a Research Letter in March 2023 that concluded smallpox vaccination did confer cross-protection to mpox.
They evaluated vaccinia virus antibodies in 162 persons ≥50 years of age in Spain; 68.5% had detectable antibodies. However, low antibody levels in 31.5% of this population indicate that addressing their vaccination should be a priority.
On July 17, 2023, The Lancet published results from a study that concluded although real-world data estimate vaccine effectiveness to be around 85%, immunological correlates of protection are still unknown.
The U.S. CDC confirmed on September 8, 2023, that the JYNNEOS vaccine is not 100% effective against initial mpox infection or secondary (breakthrough) infections.
Research efforts should be coupled with efforts to understand better the relationship between HIV and the mpox virus, including how viral suppression and CD4 counts might affect the immune response to mpox in response to vaccination, says the CDC.
Our Trust Standards: Medical Advisory Committee