U.S. CDC Committee to Review Various Vaccines

Following regulatory provisions, the U.S. Centers for Disease Control and Prevention (CDC) announced a three-day meeting of the Advisory Committee on Immunization Practices (ACIP) to begin at 8 a.m. on October 25, 2023.
The ACIP develops recommendations for immunizations, including ages when vaccines should be given, number of doses, time between doses, and precautions and contraindications.
In addition, under 42 U.S.C. 1396s, the ACIP is mandated to establish and periodically review and, as appropriate, revise the list of vaccines for administration to vaccine-eligible children through the Vaccines for Children program, along with schedules regarding dosing interval, dosage, and contraindications to administration of vaccines.
These recommendations are submitted to the CDC's Director, Mandy K. Cohen, MD, MPH, for final approval.
As of March 2023, the recently approved ACIP recommendations adopted by the CDC Director are posted on this CDC webpage.
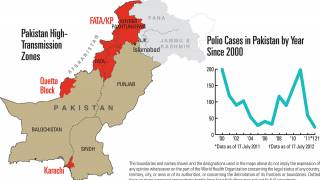
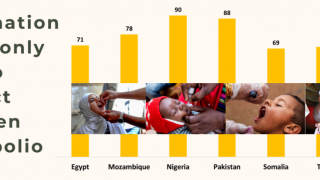
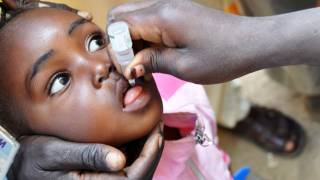
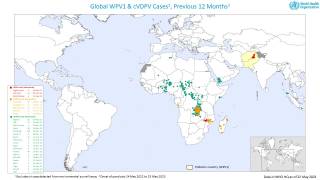
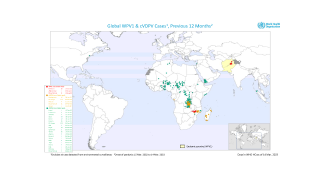
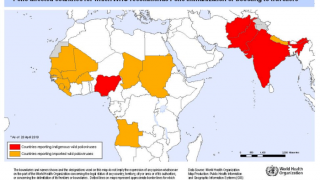
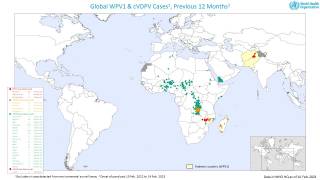
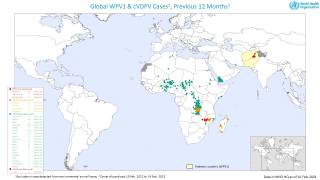
This ACIP meeting's initial agenda includes discussions and presentations on the following: child and adolescent immunization schedules, adult immunization schedules, influenza vaccines, chikungunya vaccine, COVID-19 vaccines, meningococcal vaccines, mpox vaccine, pneumococcal vaccines, polio vaccines, and, respiratory syncytial virus (RSV) vaccines for older adults.
Furthermore, recommendation votes on child and adolescent immunization schedules, adult immunization schedules, and meningococcal and mpox vaccines are scheduled.
Additionally, a Vaccines for Children vote on meningococcal and the mpox vaccines are scheduled.
Written comments from non-CDC staff must be received between October 2-13, 2023.
You may submit comments identified by Docket No. CDC-2023-0079, by Federal eRulemaking Portal: https://www.regulations.gov, or by mailing Ms. Stephanie Thomas, ACIP Meeting, Centers for Disease Control and Prevention, 1600 Clifton Road, NE, Mailstop H24-8, Atlanta, Georgia 30329-4027. Attn: Docket No. CDC-2023-0079.
For more information on ACIP, please visit the ACIP website: https://www.cdc.gov/vaccines/acip/index.html.
Our Trust Standards: Medical Advisory Committee