Mpox Vaccines
Mpox Vaccines 2024
The United States (U.S.) began offering Bavarian Nordic's JYNNEOS® vaccine to healthcare staff in Boston on May 24, 2022, followed by mpox vaccination services in New York City and San Francisco. As of 2024, over 1.2 million (1-dose: 38.8% and 2-dose: 24.3%) JYNNEOS doses had been administered in U.S. Jurisdictions. The U.S. Centers for Disease Control and Prevention (CDC) reported that the vaccine effectiveness (VE) of JYNNEOS against mpox ranges from 36 to –75% for 1-dose vaccination and 66 to –89% for 2-dose vaccination.
Mpox Vaccines
The U.S. FDA Approved the JYNNEOS® (MVA-BN, IMVANEX®, IMVAMUNE®) Smallpox and Mpox Vaccine, Live, Nonreplicating for the prevention of disease in adults on September 24, 2019. On August 3, 2023, the U.S. government ordered an additional $120 million worth of JYNNEOS vaccines. The European Medicines Agency (EMA) approved IMVANEX (Jynneos) in 2013 for preventing smallpox in the European Union (E.U.) and for mpox caused by vaccinia on July 27, 2022. The United Kingdom (U.K.) began offering IMVANEX® to specific mpox close contacts in England in May 2022. The U.K. Health Security Agency confirmed Jynneos doses remain available for gay, bisexual, and men who have sex with men at the highest risk from mpox in 2023. As of April 2024, mpox vaccination clinics are operational in London, and the JYNNEOS vaccine has begun its commercial rollout in U.S. pharmacies.
Japan's K.M. Biologics' LC16 "KMB" freeze-dried smallpox vaccine was approved as an additional indication for the prevention of the mpox virus by the Japanese Ministry of Health, Labor, and Welfare. In addition, the ACAM2000® live vaccinia virus vaccine is authorized to prevent mpox infections in various countries.
Mpox Vaccine Candidates
Tonix Pharmaceuticals Holding Corp. announced in November 2023 that the National Institute of Allergy and Infectious Diseases (NIAID) will conduct a Phase 1 clinical trial with TNX-1800 (recombinant horsepox virus, live vaccine). Tonix has received a response from a Type B pre-Investigational New Drug Application meeting with the U.S. FDA to develop TNX-801 as a potential vaccine to protect against mpox disease and smallpox. In addition, the U.S. Patent and Trademark Office issued a patent on May 31, 2022, entitled "Synthetic Chimeric Poxviruses." In July 2022, the Company announced a collaboration with the Kenya Medical Research Institute to seek regulatory approval for conducting a Phase 1 clinical study. On December 1, 2022, the Company presented the Live Virus Smallpox and Mpox Vaccine.
Beijing Institute of Biological Products Co., Ltd. (BIBP), a subsidiary of China National Biotec Group Company Limited, China National Pharmaceutical Group Co., Ltd. (Sinopharm) confirmed China's drug authority granted permission to launch a mpox vaccine candidate based on replication-defective mpox viruses that cannot spread but can grant a person immunity.
Serum Institute of India, Reliance Life Sciences, and Dr Reddy's Laboratory are among the firms collaborating on producing a vaccine for the MPox virus in India. The Indian Council of Medical Research (ICMR) began this initiative on July 27, 2022: Collaboration for developing In-Vitro Diagnostic Kits and Vaccine candidates against the MPox virus.
Emergex Vaccines Holding Limited announced on October 18, 2022, that it has formulated and confirmed the synthesis and assembly of a CD8+ T cell Adaptive Vaccine for smallpox and mpox, comprised predominantly of early "eclipse phase" antigens.
EpiVax, Inc.'s "Epitope-Driven Vaccine" is a smallpox vaccine candidate predicted to be highly effective against mpox. The development of VennVax, a DNA-prime, peptide-boost multi-T-cell epitope poxvirus vaccine, was funded by the U.S. National Institutes of Health (SBIR grant #R43AI058376).
Mpox Virus Disease
Monkeypox is a viral zoonotic virus that is transmitted from one person to another by physical contact with lesions, body fluids, respiratory droplets, and contaminated materials. The monkeypox virus causes monkeypox disease, a member of the Orthopoxvirus genus in the family Poxviridae. According to the WHO, the monkeypox virus has two distinct genetic clades: the Congo Basin and WesAfrican.
Mpox Outbreak News
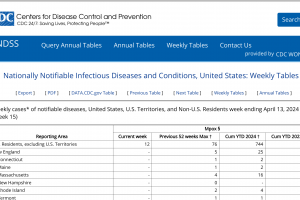
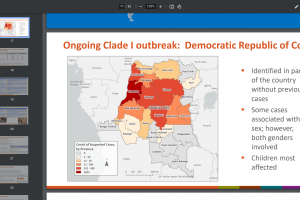
Mpox outbreak news was updated in 2024. And other sexually transmitted disease vaccine news is posted at this link.