Novel Polio Vaccine Breakthrough Cases Confirmed in Africa
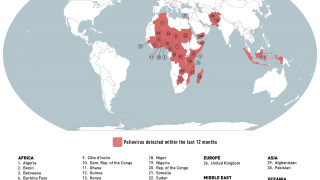
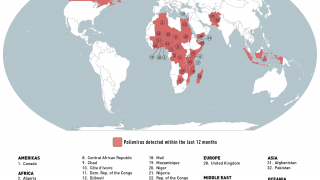
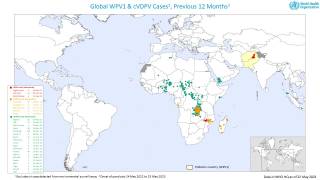
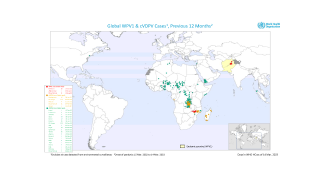
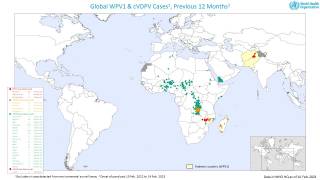
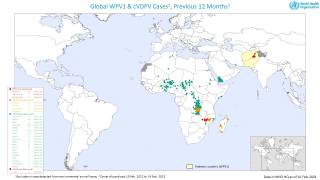
The Global Polio Eradication Initiative (GPEI) today announced it had received notification of the detection of circulating vaccine-derived poliovirus type 2 (cVDPV2) linked with the novel oral polio vaccine type 2 (nOPV2) in the African countries of Burundi and the Democratic Republic of the Congo (DRC).
These are the first instances of cVDPV2 linked with nOPV2 since the roll-out of the vaccine began in March 2021.
As of March 16, 2023, close to 600 million doses of nOPV2 have been administered across 28 countries, and most countries have seen no further transmission of cVDPV2 after two immunization rounds.
The viruses were isolated from the stool samples of seven children with acute flaccid paralysis (AFP), six in DRC (eastern Tanganyika and South Kivu provinces) and one in Burundi (Bujumbura Rural province).
And from five environmental samples collected in Burundi (Bujumbura Mairie province).
All reported isolates stem from two separate and new emergences of cVDPV2 linked with nOPV2 that originated in Tanganyika and South Kivu provinces in DRC.
The GPEI stated in a press release on March 16, 2023; it is supporting various risk assessments and vaccination responses to reduce the risk of further transmission per outbreak response protocols.
Burundi and DRC have scheduled initial vaccination campaigns for April 2023. Subsequent campaigns may be expanded to include areas in neighboring countries.
Additionally, the GPEI stated that while detecting these outbreaks is a tragedy for the families and communities affected, it is not unexpected with the broader use of the vaccine.
All available clinical and field evidence demonstrates that nOPV2 is safe and effective and has a significantly lower risk of reverting to a form that causes paralysis in low immunity settings than monovalent oral polio vaccine type 2 (mOPV2).
A preliminary assessment suggests an estimated 30-40 new cVDPV2 emergencies, conditional on surveillance inputs, would have been detected by March 1, 2023, if mOPV2 was used instead of nOPV2 at the same scale.
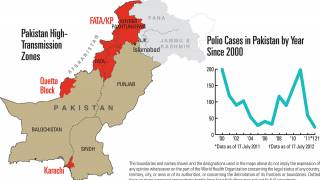
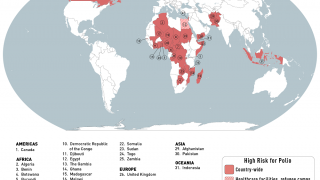
Importantly, eastern DRC is classified as one of GPEI’s seven most consequential geographies for poliovirus outbreak risk.
To warn international travelers of their risk, the World Health Organization announced in February 2023, although encouraged by the reported progress, that the risk of the global spread of poliovirus remains a Public Health Emergency of International Concern.
Complex humanitarian challenges in the country, including insecurity, have created longstanding barriers to reaching every child with the polio vaccine.
This has contributed to the continued spread of variant poliovirus within DRC and its exportation to nearby countries.
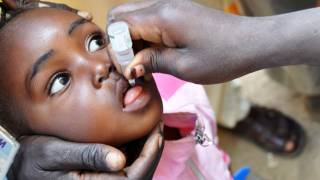
The success of nOPV2 and any polio vaccine depends on the ability to rapidly implement high-quality immunization campaigns to ensure that every child is vaccinated and the spread of poliovirus is stopped.
Ultimately, no vaccine sitting in a vial can protect a child, says the GPEI.
And the U.S. Centers for Disease Control and Prevention reissued its Alert - Level 2, Practice Enhanced Precautions notice regarding polio outbreaks on March 7, 2023.
The CDC advises that adults who previously completed the whole routine polio vaccine series before traveling to any at-risk destination receive a single, lifetime booster dose of the polio vaccine.
Various polio vaccines are available in the U.S. at health clinics and community pharmacies.
Our Trust Standards: Medical Advisory Committee