Polio Vaccine Quality Control Ensured with New Antibody Test

Human antibodies cloned by scientists at the Lankenau Institute for Medical Research (LIMR) have been adopted by the World Health Organization (WHO) as a crucial part of its plan to eradicate polio permanently.
Announced on December 9, 2022, the WHO Expert Committee on Biological Standardization approved the antibodies created in the lab of LIMR faculty member Scott Dessain, MD, Ph.D., as the standard for quality-control testing of all inactivated polio vaccines (IPV) in October 2022.
Dessain and his team tackled the issue by identifying a set of human antibodies to ensure that an IPV vaccine was made correctly.
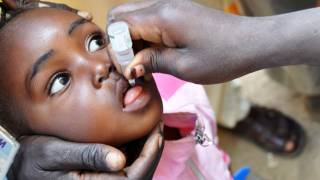
The new test will increase assurance that polio vaccines are working correctly when administered.
The committee first advanced guidelines incorporating the LIMR antibodies to test IPV potency two years ago.
"News of the New York (polio) case is a reminder of the need to continue to commit to vaccination efforts worldwide," said George Prendergast, Ph.D., president and CEO of LIMR, in a press release.
"The efforts of Dr. Dessain and his colleagues provide a key tool in quality control for the manufacture of high-potency IPV vaccines that can prevent polio outbreaks caused by mutated oral polio vaccine (OPV) across the world."
One critical difference between the two vaccine types is that, unlike IPV, OPV uses a weakened, live virus that can mutate and regain the ability to cause paralytic polio.
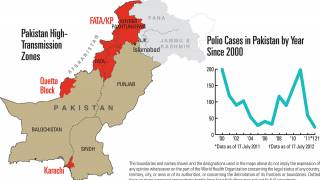
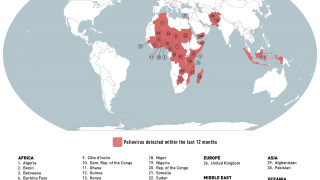
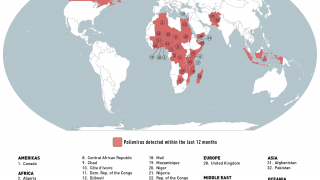
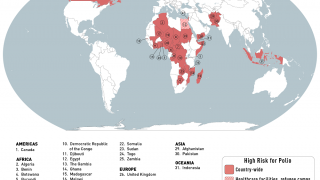
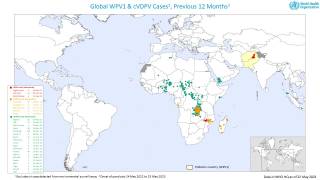
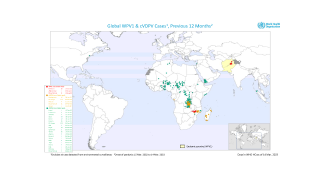
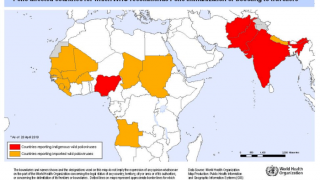
Such mutated vaccine virus strains are causing the recent global resurgence of polio cases.
In the U.S., New York state officials alerted the public in July to a polio case in Rockland County linked to OPV mutation.
As of early December 2022, about 94 wastewater samples have confirmed the presence of poliovirus in lower New York.
Furthermore, the Philadelphia Department of Public Health is exploring plans to collect wastewater samples.
"The world is beginning to transition away from the OPVs because it is the only path to ultimately eradicating polio," said Dessain, director of the Center for Human Antibody Technology at LIMR and holds the Joseph and Ray Gordon Chair in Clinical Oncology and Research.
"Making that transition meant developing a next-generation IPV and expanding production."
"But the vaccine manufacturers weren't using the same standards as one another."
"That made it necessary to find a way not only to determine the potency of the new vaccines from different manufacturers."
"But also address the problem of quality control labs not all using the same potency test."
Located in Wynnewood, PA, the LIMR opened in 1927 and was the first research center in the nation dedicated primarily to the study of cancer and the first to discover a genetic defect that contributed to human cancer, thus launching the modern era of molecular genetics in cancer research.
Other polio outbreak news is posted at PrecisionVaccinations.com/Polio.
PrecisionVaccinations publishes fact-checked, research-based vaccine information manually curated for mobile readers.
Our Trust Standards: Medical Advisory Committee