Is One HPV Vaccine Dose Enough Cancer Protection?
When the World Health Organization (WHO) updated its recommendations for the human papillomavirus (HPV) vaccine in late 2022, it stated that a single-dose schedule could provide a comparable efficacy and durability of cancer protection compared to a two-dose regimen.
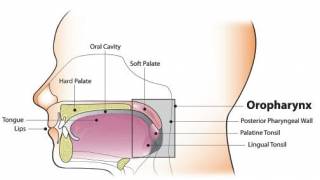
Since sexually transmitted HPV causes more than 95% of cervical cancer, many countries sought independent clinical research to support the WHO's revised recommendation.
During 2023, several research papers have been published offering insights behind the WHO's HPV vaccination schedule change.
On August 28, 2023, BMC Medicine published a research article: Global impact and cost-effectiveness of one-dose versus two-dose human papillomavirus vaccination schedules: a comparative modeling analysis that supports this one-dose schedule under certain assumptions.
Using three independent HPV transmission models, these researchers estimated the long-term health benefits and cost-effectiveness of one-dose versus two-dose HPV vaccination in 188 countries.
Under the scenarios where a single HPV vaccine dose confers more than 30 years of protection or 80% efficacy with lifelong protection, routine one-dose HPV vaccination provides the majority of health benefits to the two-dose program while simplifying vaccine delivery, reducing costs and circumventing vaccine supply constraints.
The second dose may be cost-effective if there is a shorter duration of protection from one dose, cheaper vaccine and vaccination delivery strategies, and a higher burden of cervical cancer.
These modeling results were fairly consistent when projected from three independent transmission dynamic models used in nine countries.
The outcomes of this comparative modeling analysis contribute to the extensive evidence base, including emerging evidence from the single-dose HPV vaccine trials and observational studies, which would benefit policymakers when they consider HPV vaccination in their populations, wrote these researchers.
"The HPV vaccine is highly effective for the prevention of HPV serotypes 16 & 18, which cause 70% of cervical cancer," said Dr. Alejandro Cravioto, WHO SAGE Chair, in a press release in 2022.
"SAGE urges all countries to introduce HPV vaccines and prioritize multi-age cohort catch-up of missed and older cohorts of girls."
"These recommendations will enable more girls and women to be vaccinated and thus prevent them from having cervical cancer and all its consequences throughout their lifetimes."
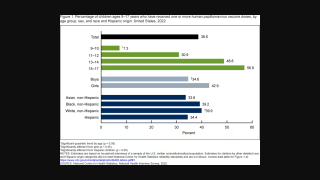
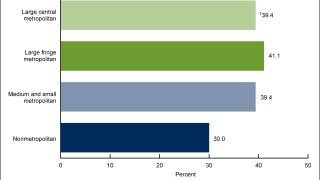
In the U.S., HPV accounts for about 14 million new infections annually.
The Centers for Disease Control and Prevention (CDC) updated its HPV vaccination schedule in 2023. The CDC recommends routine vaccination of preteens at ages 11 or 12 years. The vaccination series can be started at age nine years, if appropriate.
And HPV vaccinations may be given simultaneously with other vaccines, says the CDC.
Various HPV vaccines are available in 2023 in the U.S. at health clinics and pharmacies and globally.
Our Trust Standards: Medical Advisory Committee
- Global impact and cost-effectiveness of one-dose versus two-dose HPV vaccination schedules: a comparative model
- A single dose of quadrivalent HPV vaccine is highly effective against HPV genotypes 16 and 18 detection in young pregnant women
- Human papillomavirus vaccines: WHO position paper, December 2022