HPV Vaccinations Can Prevent Anal Cancer

A large clinical trial of people living with HIV has found that treating anal precancerous growths known as high-grade squamous intraepithelial lesions (HSIL) reduces the chance that anal cancer will develop by over 50%.
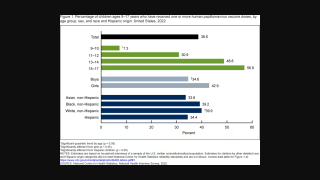
Nearly all cases of anal cancer are caused by infection of anal cells with cancer-causing types of human papillomavirus (HPV).
Although HPV vaccination can prevent new anal HPV infections, no evidence-based recommendations are currently available to guide screening for and treating anal HSIL.
Results from the Anal Cancer/HSIL Outcomes Research (ANCHOR) study were published on June 16, 2022, as an Original Article by the New England Journal of Medicine peer-reviewed journal.
With a median follow-up of 25.8 months, 9 cases were diagnosed in the treatment group and 21 in the active-monitoring group. The rate of progression to anal cancer was lower in the treatment group than in the active-monitoring group by 57% (95% CI, 6 to 80; P=0.03 by log-rank test).
“We've now shown for the first time that treating anal HSIL is effective at reducing the incidence of anal cancer in a very high-risk group of people — people living with HIV,” commented Joel Palefsky, M.D., of the University of California, San Francisco, in a press statement.
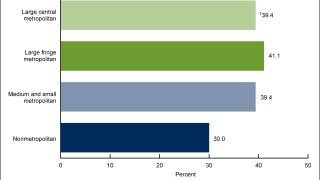
Anal cancer is relatively rare in the general population, the incidence of anal cancer in the general population is 1.6 per 100,000.
However, it is the fourth most common cancer among people living with HIV. The incidence of anal cancer is estimated to be 89 per 100,000 among men living with HIV who have sex with men and between 18.6 and 35.6 per 100,000 among women living with HIV.
If the immune system does not fight off the infection, the lingering infection can cause the cells to become progressively more abnormal, ultimately developing into HSIL and, in some cases, into anal cancer, stated these researchers.
This progression is similar to what is seen in cervical cancer, which is also caused by HPV.
Routine screening for cervical cancer with HPV and/or Pap testing and removing cervical HSIL has been shown to prevent many cases of cervical cancer. However, it has been unclear whether the treatment of anal HSIL found through screening similarly prevents anal cancer.
Moreover, the treatment of anal HSIL is more challenging than the treatment of cervical HSIL, and recurrences are common.
The ANCHOR study is the first randomized controlled trial to determine whether treating anal HSIL is a safe and effective strategy for reducing the progression of HSIL to anal cancer. The National Cancer Institute, part of the National Institutes of Health, funded the trial. No industry conflicts of interest were disclosed.
PrecisionVaccinations publishes fact-checked, research-based HPV vaccine news curated for mobile readership.
Our Trust Standards: Medical Advisory Committee
- Treatment of Anal High-Grade Squamous Intraepithelial Lesions to Prevent Anal Cancer
- Topical or Ablative Treatment in Preventing Anal Cancer in Patients With HIV and Anal High-Grade Squamous Intraepithelial Lesion
- In people with HIV, treating precancerous anal lesions cuts risk of anal cancer by more than half
- HPV Vaccines