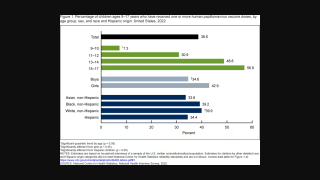
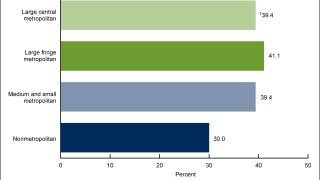
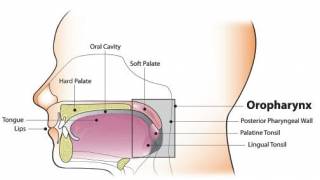
HPV Vaccine Booster Could Reduce Cervical Lesions

While the introduction of human papillomavirus (HPV) vaccines has revolutionized cervical cancer prevention, related diseases have not been eliminated.
New research on the role of HPV vaccination offers keen insights into the recurrence of HPV-related diseases in women undergoing surgical treatment.
Women who develop high-grade cervical intraepithelial neoplasia (CIN) are susceptible to infection with HPV and can rapidly re-acquire infections after local surgical treatment.
Published by The British Medical Journal on August 5, 2022, this systematic review and meta-analysis found that to help reduce precancerous cervical lesions, an injection of the new HPV vaccine during surgery may help prevent future lesions.
The primary outcome measure was the risk of recurrence of cervical intraepithelial neoplasia grade 2 or higher (CIN2+) after local surgical treatment.
The analysis showed the risk of recurrence of high-grade, pre-invasive disease was reduced by 57% in vaccinated women.
And this vaccination benefit was most observable when related to HPV16 or HPV18 infections.
These HPV-vaccinated women saw a 74% reduction in precancerous lesions.
However, evidence was lacking on the benefit of HPV vaccination for recurrence of vulvar, vaginal, or anal intraepithelial lesions, genital warts, or for incident or persistent HPV infections, although the data were scarce with small numbers of studies and participants.
These researchers concluded that 'Women who have been treated for high-grade CIN have a lifelong residual high risk of cervical cancer and other malignancies related to HPV infection.'
New strategies to reduce the risk in these women might shorten and simplify screening programs and eliminate cervical cancer more rapidly.
But, large, appropriately powered randomized controlled trials are required to establish the effectiveness of adjuvant HPV vaccination at the time of local surgical treatment of CIN.
Given that the incidence of recurrence of high-grade disease is low in quality assured national screening programs, such as in the UK, absolute risks and a cost-effectiveness analysis would be important in determining the implementation strategy of HPV vaccination after treatment.
The NOVEL (Nonavalent Prophylactic HPV Vaccine (Gardasil 9) After Local Conservative) trial, an ongoing, large, appropriately powered randomized controlled trial, is expected to report results and give further insight on the effect of Gardasil9 on the persistent incident, recurrent, and prevalent HPV infections and cost-effectiveness.
The Gardasil9 vaccine consists of HPV proteins, Types 6, 11, 16, 18, 31, 33, 45, 52, and 58.
If the study reports benefit, the results could apply to women with multifocal disease, other diseases related to HPV infection, and individuals with a diagnosis of malignancies associated with HPV infection.
In total, 22 articles met the review's inclusion criteria; 18 of these studies also reported data from a non-vaccinated group and were included in the meta-analyses (12 observational studies, two randomized controlled trials, and four post hoc analyses of randomized controlled trials).
This work was supported by the National Institute for Health and Care Research, the Imperial Healthcare NHS Trust NIHR Biomedical Research Centre, and others. These researchers did not disclose industry-related conflicts of interest.
Additional HPV vaccine news is posted at PrecisionVaccinations.com/HPV.
PrecisionVaccinations publishes fact-checked, research-based vaccine news curated for mobile readership.
Updated on Jan. 19, 2023 for the picture.
Our Trust Standards: Medical Advisory Committee