New Dengue Therapeutic Coming Soon
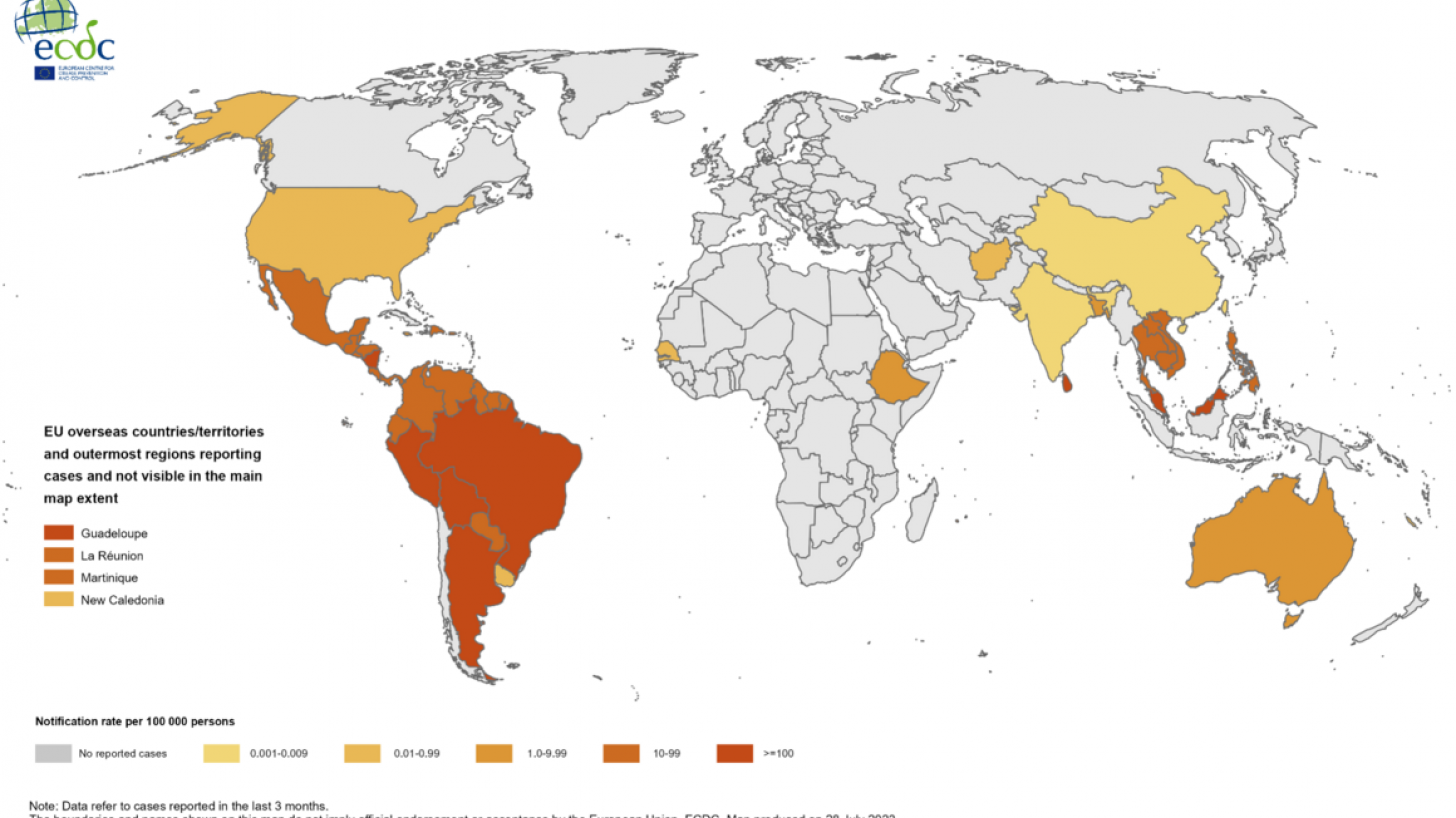
Dengue outbreaks have steadily increased in the last 50 years in East Asia countries, and 2023 is no exception. This year, tens of thousands of dengue cases have been diagnosed in India.
And about half of India's population already carry dengue virus-specific antibodies.
While two dengue virus (DENV) vaccines are approved in specific countries, and several vaccine candidates are conducting late-stage clinical trials, India may soon launch an innovative monoclonal antibody (mAb) that immediately delivers passive immunity.
An obstacle to the development of mAb therapeutics against DENV has been the identification of antibodies capable of cross-neutralizing Dengue's four viruses.
Furthermore, monovalent mAb therapeutics risk becoming obsolete if viral immune evasion results in the loss of neutralizing epitopes.
Asia's largest vaccine maker, Serum Institute of India Pvt Ltd (SIIPL), recently confirmed it is tackling these risks by codeveloping a biological therapy to treat all four strains of the dengue virus.
SIIPL is collaborating with a U.S.-based Visterra to deploy its VIS513 humanized mAb to treat Dengue. In vitro and preclinical animal models have shown VIS513 to be a highly potent inhibitor of dengue viruses.
Serum recently confirmed it would soon apply to India's Ministry of Science and Technology for a fast-track approval.
SIIPL's CEO Poonawalla commented on his website, "We expect the government to give a fast-track approval to the application. This is because Dengue has become a public health crisis. We would initiate clinical trials after approval to prove its safety and efficacy."
Poonawalla added, "The VIS513 had shown promising results according to the "animal mode" studies and has worked to neutralize the four strains of dengue virus."
"If the trials bear positive results, Serum could be the pioneer in offering a cure for the dangerous infection which has probably had the worst outbreak this year."
The mAb would be a single injection costing 5,000 to 10,000 rupees per dose.
Serum holds exclusive commercial licenses in India, Pakistan, Bangladesh, Nepal, Bhutan, Maldives, and Sri Lanka. And would be held accountable for the clinical development and funding of VIS513 in the Indian Subcontinent.
Our Trust Standards: Medical Advisory Committee
























