Qdenga Targets 100 Million Vaccine Production in 2030

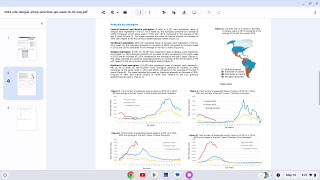
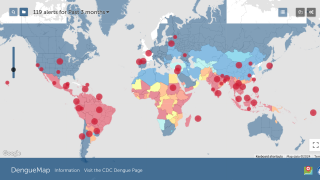
The global dengue outbreak continues to accelerate in over 100 endemic areas this year. In 2023, there was an increase of over 30% in dengue cases and related deaths compared to 2022.
And some regions were impacted more than others.
For instance, the Region of the Americas reported 4,565,911 dengue cases, leading to 2,340 deaths.
Unfortunately, tens of thousands of people are living unprotected and unable to combat this mosquito-transmitted disease.
However, recent news indicates that more people will soon have access to a second-generation, safe dengue vaccine.
Biological E. Limited (BE) and Takeda announced on February 26, 2024, that a strategic partnership will accelerate access to Qdenga® (Dengue Tetravalent Vaccine [Live, Attenuated]) multi-dose vials (MDVs).
MDVs offer economic and logistical advantages for National Immunization Programs by minimizing packaging and storage expenses and reducing medical and environmental waste.
BE has committed to ramping up to a manufacturing capacity of up to 50 million doses a year, accelerating Takeda's efforts to manufacture 100 million doses annually within the decade.
These doses will ultimately be made available for procurement by governments in endemic countries by 2030 at the latest.
"We are proud to partner with BE to significantly increase our supply of Qdenga, accelerating our efforts to reach a total manufacturing capacity of 100 million doses a year by 2030, at the latest," said Gary Dubin, M.D., president of the Global Vaccine Business Unit at Takeda, in an email to Precision Vaccinations.
"This collaboration will make multi-dose vials which will ultimately be available for procurement by governments in dengue-endemic countries to support National Immunization Programs."
"Multi-dose vials have economic and logistical benefits when used in large public vaccination programs since they can be packaged and stored more efficiently, reducing program costs and facilitating their implementation."
As of January 2024, the two-dose Qdenga vaccine was launched in 21 countries, and this innovative dengue vaccine does not require pre-vaccination testing.
However, Qdenga is unavailable in dengue-endemic areas, such as Costa Rica, Puerto Rico, and southeast Florida.
To alert international travelers to their risk of dengue, the U.S. Centers for Disease Control and Prevention (CDC) says dengue is a growing health threat globally, with multiple factors potentially contributing to the increasing incidence and expansion into new areas, including rapid urbanization and increased travel.
Dengue outbreaks can be abrupt and strain health care systems, requiring additional interventions and resources, including vaccines and effective and scalable vector control methods to reduce dengue morbidity and mortality.
The CDC has issued Travel Health Notices regarding dengue outbreaks in the Americas (Feb. 9, 2024), Asia/Pacific Islands (Feb. 9, 2024), and Africa/Middle East (Feb. 16, 2024).
Our Trust Standards: Medical Advisory Committee